Get the free 7-Day Premarket Notification for New Dietary Ingredients - fda
Show details
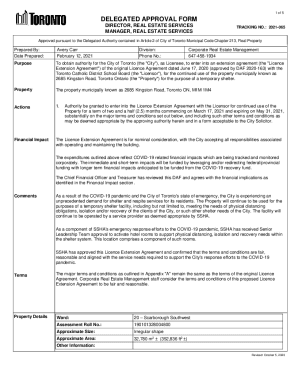
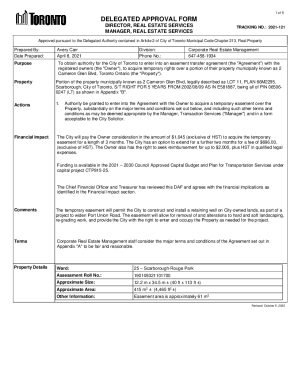
This document serves as a notification to the FDA regarding the introduction of a new dietary ingredient, heme iron polypeptide, by Colorado Biolabs, Inc., including safety assessments and relevant
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign 7-day premarket notification for

Edit your 7-day premarket notification for form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your 7-day premarket notification for form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing 7-day premarket notification for online
To use our professional PDF editor, follow these steps:
1
Check your account. It's time to start your free trial.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit 7-day premarket notification for. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, dealing with documents is always straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out 7-day premarket notification for

How to fill out 7-Day Premarket Notification for New Dietary Ingredients
01
Gather all necessary information about the new dietary ingredient (NDI), including its identity, composition, and intended use.
02
Prepare a detailed description of the dietary ingredient and its production process.
03
Include information on the history of the ingredient and any previous food or supplement uses.
04
Compile any safety data or toxicological studies that demonstrate the ingredient is safe under the conditions of use.
05
Fill out the 7-Day Premarket Notification form accurately, ensuring all sections are completed with the required details.
06
Submit the completed notification to the FDA through the appropriate channels, ensuring compliance with the submission guidelines.
Who needs 7-Day Premarket Notification for New Dietary Ingredients?
01
Manufacturers and marketers of dietary supplements that intend to introduce new dietary ingredients into the market.
02
Companies seeking to ensure compliance with FDA regulation regarding the safety and premarket notification of new dietary ingredients.
Fill
form
: Try Risk Free






People Also Ask about
When must a manufacturer or distributor notify FDA about a dietary supplement it is going to market?
The notification must be submitted to FDA at least 75 days before introducing the product into interstate commerce or delivering it for introduction into interstate commerce.
What is considered a dietary ingredient?
The term "dietary ingredient" includes vitamins and minerals; herbs and other botanicals; amino acids; "dietary substances" that are part of the food supply, such as enzymes and live microbials (commonly referred to as "probiotics"); and concentrates, metabolites, constituents, extracts, or combinations of any dietary
When must a manufacturer or distributor notify the FDA about a dietary supplement it intends to market in the United States?
Either the manufacturer or distributor of a dietary supplement that contains an NDI, or the manufacturer or distributor of the NDI, must notify FDA at least 75 days before marketing the article in the United States, unless the NDI has been present in the food supply as an article used for food in a form in which the
What is a new dietary ingredient notification master file for dietary supplements?
For purposes of the guidance, a new dietary ingredient notification Master File (NDIN Master File or Master File) is a file containing identity, manufacturing, and/or safety information relating to a new dietary ingredient (NDI) that the Master File owner submits to FDA for use in evaluating a potential future NDIN by
What is a new dietary ingredient notification master file for dietary supplements?
For purposes of the guidance, a new dietary ingredient notification Master File (NDIN Master File or Master File) is a file containing identity, manufacturing, and/or safety information relating to a new dietary ingredient (NDI) that the Master File owner submits to FDA for use in evaluating a potential future NDIN by
What is a new dietary ingredient notification?
A New Dietary Ingredient Notification (NDIN) is a notification that must be submitted to the FDA for a dietary ingredient that was not marketed in the U.S. as a dietary supplement before October 15, 1994.
What are the requirements of manufacturers to market a dietary supplement?
Federal Regulation of Dietary Supplements Medicines must be approved by FDA before they can be sold or marketed. Supplements do not require this approval. Supplement companies are responsible for having evidence that their products are safe, and the label claims are truthful and not misleading.
What does "new dietary ingredient" mean?
What is a "new dietary ingredient?" The term "new dietary ingredient" means a dietary ingredient that was not marketed in the United States in a dietary supplement before October 15, 1994. (See section 413(d) of the Federal Food, Drug, and Cosmetic Act (the FD&C Act), 21 U.S.C.
How many days in advance must a distributor inform the FDA before bringing a nutritional supplement to market?
The notification must be submitted to FDA at least 75 days before introducing the product into interstate commerce or delivering it for introduction into interstate commerce.
Are dietary supplements subject to pre market safety regulations by the FDA?
Yes. The manufacturer or distributor of a new dietary ingredient or of a dietary supplement that contains a new dietary ingredient must submit a notification to FDA at least 75 days before introducing or delivering for introduction into interstate commerce a dietary supplement that contains the new dietary ingredient.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is 7-Day Premarket Notification for New Dietary Ingredients?
The 7-Day Premarket Notification for New Dietary Ingredients is a submission to the FDA that must be made by manufacturers or distributors of dietary supplements containing new dietary ingredients (NDIs) that were not marketed in the U.S. before October 15, 1994. This notification informs the FDA about the safety of the NDI and must be filed at least 7 days before the product is marketed.
Who is required to file 7-Day Premarket Notification for New Dietary Ingredients?
Manufacturers or distributors of dietary supplements who plan to market a product containing a new dietary ingredient that was not marketed before October 15, 1994 are required to file the 7-Day Premarket Notification with the FDA.
How to fill out 7-Day Premarket Notification for New Dietary Ingredients?
To fill out the 7-Day Premarket Notification, manufacturers or distributors must provide information about the new dietary ingredient, including its identity, the quantity proposed for use, the conditions of use, evidence of safety, and a declaration indicating whether the ingredient was marketed before the cutoff date. The notification can be submitted via mail or electronically through the FDA's digital submission system.
What is the purpose of 7-Day Premarket Notification for New Dietary Ingredients?
The purpose of the 7-Day Premarket Notification is to ensure that new dietary ingredients are safe for consumption before they can be marketed to the public. It allows the FDA to assess the safety and validity of the new ingredient based on scientific evidence provided by the manufacturer or distributor.
What information must be reported on 7-Day Premarket Notification for New Dietary Ingredients?
The information that must be reported includes the name of the new dietary ingredient, a description of the intended use, the quantity to be used in the product, any relevant safety information, and any evidence that supports the conclusion that the ingredient is safe. Additional relevant data regarding the source and manufacturing practices may also be included.
Fill out your 7-day premarket notification for online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

7-Day Premarket Notification For is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.