Get the free Application for Biopharma Certificate Program and/or for Non-Matriculation Status - ...
Show details
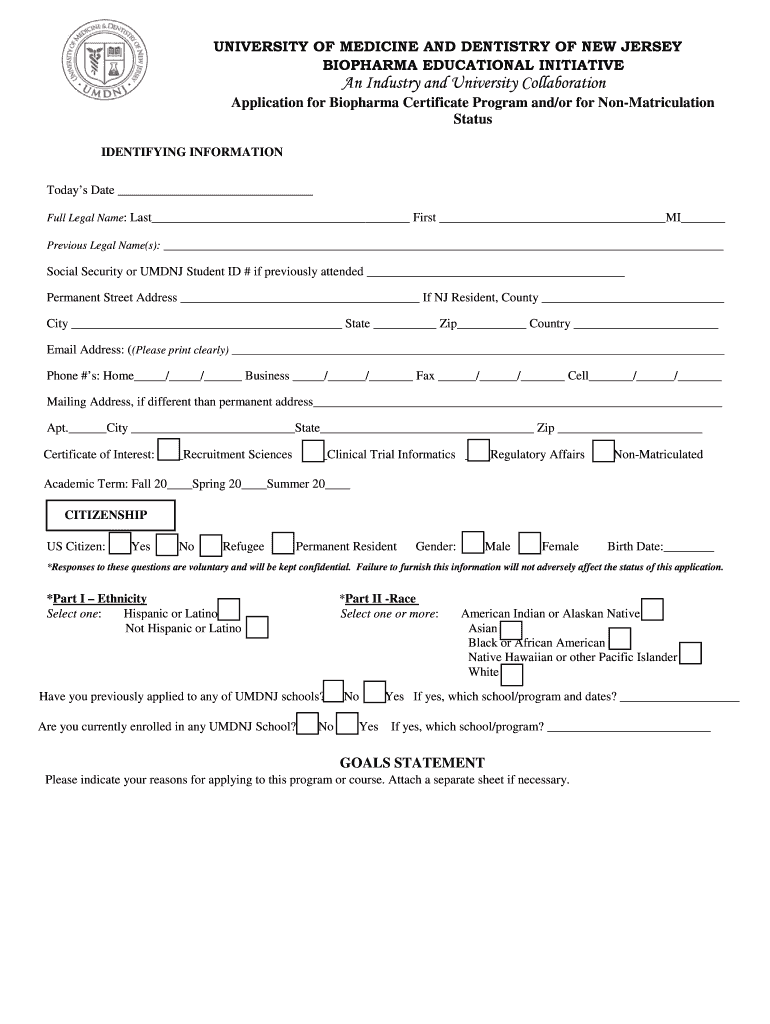
This document serves as an application form for the Biopharma Certificate Program at the University of Medicine and Dentistry of New Jersey, collecting identifying information and academic history
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign application for biopharma certificate

Edit your application for biopharma certificate form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your application for biopharma certificate form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing application for biopharma certificate online
To use the services of a skilled PDF editor, follow these steps:
1
Log in to your account. Start Free Trial and register a profile if you don't have one.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit application for biopharma certificate. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
Dealing with documents is simple using pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out application for biopharma certificate

How to fill out Application for Biopharma Certificate Program and/or for Non-Matriculation Status
01
Visit the official website of the Biopharma Certificate Program.
02
Locate the section for the Application Form.
03
Download the Application for Biopharma Certificate Program or the Non-Matriculation Status Application.
04
Carefully read the instructions for filling out the application.
05
Provide your personal information, including your name, address, and contact details.
06
Fill out your educational background and any relevant experience in the field of biopharma.
07
Attach any required documents, such as transcripts or a resume.
08
Review your application for any errors or missing information.
09
Submit the completed application form by the given deadline.
Who needs Application for Biopharma Certificate Program and/or for Non-Matriculation Status?
01
Individuals seeking to further their education in biopharma without enrolling in a degree program.
02
Professionals looking to gain specific knowledge or skills relevant to the biopharma industry.
03
Students interested in a certificate program to enhance their qualifications.
04
Anyone wishing to enroll in courses related to biopharma on a non-matriculated basis.
Fill
form
: Try Risk Free






People Also Ask about
What is biopharmaceutical regulatory affairs?
The regulatory affairs (RA) department of a pharmaceutical company is responsible for obtaining approval for new pharmaceutical products and ensuring that approval is maintained for as long as the company wants to keep the product on the market.
What is regulatory affairs in pharmaceuticals?
Regulatory affairs is a multidisciplinary field that revolves around ensuring that health-related products, including pharmaceuticals, medical devices, biotechnology, and biologics, comply with the guidelines set forth by regulatory agencies.
What is the job description of regulatory affairs?
What does a regulatory affairs specialist do? A regulatory affairs specialist is responsible for ensuring that every product their company sells meets relevant government legislation, and that patient safety and efficacy are at the forefront of business activities.
Which certification is best for the pharmaceutical industry?
Certified Pharmaceutical Industry Professional (CPIP) The Certified Pharmaceutical Industry Professional (CPIP) credential is a professional certification program for the pharmaceutical industry covering product development through manufacturing.
What are biopharmaceutical regulatory affairs?
The regulatory affairs (RA) department of a pharmaceutical company is responsible for obtaining approval for new pharmaceutical products and ensuring that approval is maintained for as long as the company wants to keep the product on the market.
What is the highest salary for a regulatory affairs specialist?
The average Regulatory Affairs Specialist salary in the United States is $211,094 as of May 01, 2025. The range for our most popular Regulatory Affairs Specialist positions (listed below) typically falls between $62,639 and $359,548.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Application for Biopharma Certificate Program and/or for Non-Matriculation Status?
The Application for Biopharma Certificate Program and/or for Non-Matriculation Status is a formal request for enrollment in a specific biopharmaceutical training program, allowing individuals to gain specialized knowledge and credentials within the industry without committing to a full degree program.
Who is required to file Application for Biopharma Certificate Program and/or for Non-Matriculation Status?
Individuals interested in pursuing the Biopharma Certificate Program or those who wish to take courses without enrolling in a degree program are required to file this application.
How to fill out Application for Biopharma Certificate Program and/or for Non-Matriculation Status?
To fill out the application, applicants should provide personal information, details regarding their educational background, any relevant work experience, and specify the courses they wish to enroll in.
What is the purpose of Application for Biopharma Certificate Program and/or for Non-Matriculation Status?
The purpose is to assess the qualifications of the applicant for entering the Biopharma Certificate Program or to allow individuals to take courses on a non-matriculated basis for professional development.
What information must be reported on Application for Biopharma Certificate Program and/or for Non-Matriculation Status?
Applicants must report personal identification details, educational history, any relevant professional experience, and specific courses they are interested in attending.
Fill out your application for biopharma certificate online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Application For Biopharma Certificate is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















