Get the free Retrospective Protocol Violation Report - webmedia unmc
Show details
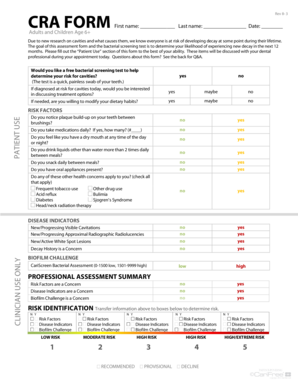
This document outlines the procedure for reporting retrospective protocol violations in research studies, ensuring compliance with regulatory standards and protection of research subjects.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign retrospective protocol violation report

Edit your retrospective protocol violation report form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your retrospective protocol violation report form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit retrospective protocol violation report online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit retrospective protocol violation report. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Create an account to find out for yourself how it works!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out retrospective protocol violation report

How to fill out Retrospective Protocol Violation Report
01
Obtain a copy of the Retrospective Protocol Violation Report form.
02
Read the instructions carefully to understand the requirements.
03
Fill in the participant's identification details, including their name, ID number, and contact information.
04
Describe the protocol violation in detail, including the date and nature of the violation.
05
Provide any relevant evidence or documentation that supports the report.
06
Fill in the corrective actions taken or proposed to address the violation.
07
Include your name, title, and signature as the person reporting the violation.
08
Submit the completed report to the designated authority as outlined in the instructions.
Who needs Retrospective Protocol Violation Report?
01
Research staff involved in clinical trials.
02
Institutional Review Boards (IRBs) for oversight purposes.
03
Regulatory bodies for compliance with research standards.
04
Sponsors of the research studies for monitoring and evaluation.
Fill
form
: Try Risk Free






People Also Ask about
Is a protocol violation the same as a protocol deviation?
A protocol violation is a subset of protocol deviation. It is any planned or intended change or deviation from the IRB approved study protocol, consent document, recruitment process, or study materials that were not approved by the IRB prior to implementation.
What is an example of a protocol violation?
Examples of minor protocol violations include, but are not limited to: Routine safety lab work for a participant without new clinical concerns and a history of previously normal lab values is inadvertently omitted at a study visit or performed outside the protocol-defined window.
Which of the following is not a type of protocol?
Round-robin protocol is not a type of protocol that determines which device can transmit on a con
Which of the following is not an example of a protocol?
Community Answer LAN is not a protocol. Whereas FTP, IP, and SAP are examples of protocols used in data communication, LAN is a type of network structure.
Do you need IRB approval for retrospective chart review?
Yes you should have IRB approval for anything not being used internally-only, and even then it's good practice to get IRB approval. Some journals ask for your approval letter. You can expect an expedited review with exemption from consent since it's retrospective so should be fairly easy to get approval.
Which of the following is not a good example of protocol violation?
The correct answer is A. The Research Coordinator consistently arriving late to study visits would not be a good example of a protocol violation, as it does not directly involve the study procedures or participant treatment.
Which is not a good example of protocol violation?
Explanation: The correct answer is A. The Research Coordinator consistently arriving late to study visits would not be a good example of a protocol violation, as it does not directly involve the study procedures or participant treatment.
What is an example of a protocol violation?
Examples of minor protocol violations include, but are not limited to: Routine safety lab work for a participant without new clinical concerns and a history of previously normal lab values is inadvertently omitted at a study visit or performed outside the protocol-defined window.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Retrospective Protocol Violation Report?
A Retrospective Protocol Violation Report is a document used to record and analyze deviations from the established research protocol after the study has concluded, ensuring transparency and accountability.
Who is required to file Retrospective Protocol Violation Report?
Typically, principal investigators, study coordinators, or any authorized personnel involved in the management of the research study are required to file the report.
How to fill out Retrospective Protocol Violation Report?
To fill out the report, users should provide details such as the nature of the violation, date of occurrence, the impact on the study, corrective actions taken, and signatures of responsible individuals.
What is the purpose of Retrospective Protocol Violation Report?
The purpose is to document protocol deviations for oversight, improve future compliance, ensure integrity of the research findings, and maintain ethical standards in research.
What information must be reported on Retrospective Protocol Violation Report?
Key information includes the description of the violation, date and time, individuals involved, potential effects on the study results, corrective actions taken, and any additional relevant notes.
Fill out your retrospective protocol violation report online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Retrospective Protocol Violation Report is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.