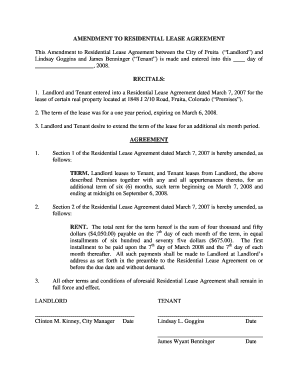
Get the free SN1 and E1 Reactions Lecture - amherst
Show details
Lecture notes covering the stability of carbocations, mechanisms of SN1 and E1 reactions, and solvent effects on reaction rates.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign sn1 and e1 reactions

Edit your sn1 and e1 reactions form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your sn1 and e1 reactions form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit sn1 and e1 reactions online
To use our professional PDF editor, follow these steps:
1
Log into your account. In case you're new, it's time to start your free trial.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit sn1 and e1 reactions. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out sn1 and e1 reactions

How to fill out SN1 and E1 Reactions Lecture
01
Start by reviewing the definitions of SN1 and E1 reactions.
02
Outline the basic mechanisms of both SN1 and E1 reactions, emphasizing the formation of carbocations.
03
List the characteristics of substrates that favor SN1 and E1 reactions, such as tertiary carbons for SN1.
04
Explain the role of nucleophiles in SN1 reactions and the role of bases in E1 reactions.
05
Provide examples of SN1 and E1 reactions with detailed mechanisms.
06
Discuss the factors affecting the rate of SN1 and E1 reactions, such as solvent effects and stability of intermediates.
07
Summarize key differences between SN1 and E1 reactions, such as the type of reaction (nucleophilic substitution vs. elimination).
08
Include practice problems or question prompts for students to apply their knowledge.
Who needs SN1 and E1 Reactions Lecture?
01
Chemistry students studying organic reactions.
02
Students preparing for exams in organic chemistry.
03
Researchers who need a refresher on reaction mechanisms.
04
Educators teaching organic chemistry topics.
05
Laboratory technicians working with organic synthesis.
Fill
form
: Try Risk Free






People Also Ask about
What is the substitution reaction?
Comparing E1 and E2 mechanisms 1) The base: strong bases favor the E2 mechanism, whereas, E1 mechanisms only require a weak base. 2) The solvent: good ionizing xolvents (polar protic) favor the E1 mechanism by stabilizing the carbocation intermediate.
What is the difference between E1 and s1 reaction?
The alcohol is the product of an SN1 reaction and the alkene is the product of the E1 reaction. The characteristics of these two reaction mechanisms are similar, as expected. They both show first order kinetics; neither is much influenced by a change in the nucleophile/base; and both are relatively non-stereospecific.
How to know if SN1 or E1 is favored?
Comparing the E1 vs SN1 Reactions With tertiary alckyl halides, E1 will generally be favored over SN1 when heat is applied. Secondly, in E1 reactions of alcohols where acid is added, the E1 is favored when the counter-ion of the acid is a poor nucleophile (e.g. H2SO4, H3PO4, TsOH)
What are SN1 and E1 reactions?
The Sn1 mechanism leads to substitution products, and the E1 mechanism leads to formation of alkenes.
What is elimination and substitution reactions?
An elimination reaction is a type of chemical reaction where several atoms either in pairs or groups are removed from a molecule. The removal usually takes place due to the action of acids and bases or the action of metals. It can also happen through the process of heating at high temperatures.
What is the elimination reaction?
What is Substitution Reaction? The substitution reaction is defined as a reaction in which the functional group of one chemical compound is substituted by another group or it is a reaction which involves the replacement of one atom or a molecule of a compound with another atom or molecule.
What is elimination and substitution reaction?
Elimination means removal. So, a reaction in which only the removal of atoms takes place is called an elimination reaction. Substitution means replacing one thing with another. Such a reaction, in which an atom or group is replaced by other atoms is called a substitution reaction.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is SN1 and E1 Reactions Lecture?
SN1 and E1 Reactions Lecture covers the mechanisms and kinetics of nucleophilic substitution (SN1) and elimination (E1) reactions in organic chemistry, emphasizing the factors affecting these processes.
Who is required to file SN1 and E1 Reactions Lecture?
Students or professionals studying organic chemistry or related fields may need to understand or submit assignments related to SN1 and E1 reactions as part of their curriculum.
How to fill out SN1 and E1 Reactions Lecture?
To fill out SN1 and E1 Reactions Lecture, one should include clear explanations of the reaction mechanisms, relevant examples, diagrams illustrating the processes, and important notes on reaction conditions.
What is the purpose of SN1 and E1 Reactions Lecture?
The purpose of the SN1 and E1 Reactions Lecture is to educate learners about these specific organic reactions, helping them grasp how they occur, their applications, and their significance in chemical processes.
What information must be reported on SN1 and E1 Reactions Lecture?
The lecture should report information including reaction mechanisms, factors influencing the reactions, kinetics, typical substrates, and examples that illustrate the concepts effectively.
Fill out your sn1 and e1 reactions online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

sn1 And e1 Reactions is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.