Get the free Event Requiring Prompt Reporting to the IRB - nyu
Show details
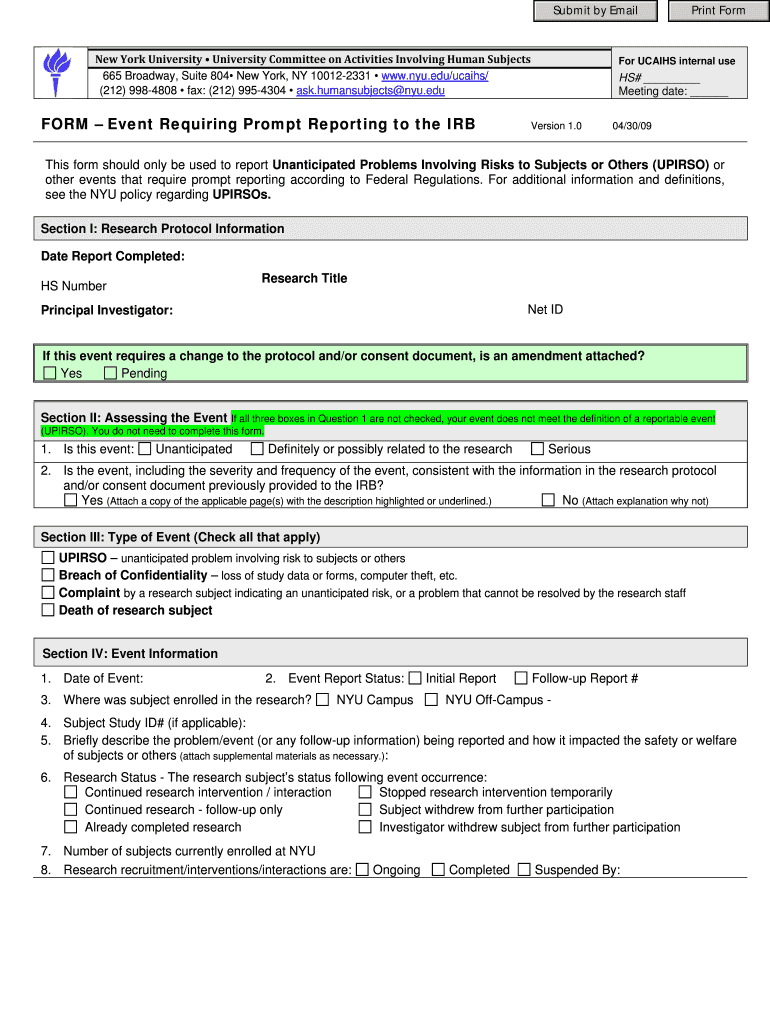
This form is used to report unanticipated problems involving risks to research subjects, which require prompt reporting according to federal regulations.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign event requiring prompt reporting

Edit your event requiring prompt reporting form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your event requiring prompt reporting form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit event requiring prompt reporting online
Follow the guidelines below to benefit from the PDF editor's expertise:
1
Log in to your account. Click Start Free Trial and sign up a profile if you don't have one yet.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit event requiring prompt reporting. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
With pdfFiller, dealing with documents is always straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out event requiring prompt reporting

How to fill out Event Requiring Prompt Reporting to the IRB
01
Begin by obtaining the Event Requiring Prompt Reporting form from the IRB's website or office.
02
Fill out the basic information section, including the study title, principal investigator, and date of the event.
03
Describe the event in detail, including what occurred, when it occurred, and who was involved.
04
Assess the event's impact on participant safety and study integrity, and indicate this in the relevant section.
05
Include any steps taken to address the event or prevent recurrence.
06
Sign and date the form, ensuring that all required signatures are obtained.
07
Submit the completed form to the IRB according to the provided guidelines.
Who needs Event Requiring Prompt Reporting to the IRB?
01
All researchers conducting studies involving human participants.
02
Study sponsors and regulatory personnel involved in oversight.
03
Institutional Review Board (IRB) members for review purposes.
Fill
form
: Try Risk Free






People Also Ask about
What must be reported immediately to the IRB?
Response: Unanticipated adverse device effect. New or increased risk. Protocol deviation that harmed a subject or placed subject at risk of harm. Protocol deviation made without prior IRB approval to eliminate an immediate hazard to a subject. Audit, inspection, or inquiry by a federal agency.
What type of adverse events must be reported to the IRB?
Adverse events that are serious, unexpected, and related or possibly related to participation in the research. Serious adverse events that are expected in some subjects, but are determined to be occurring at a significantly higher frequency or severity than expected.
What are IRB reportable events?
To ensure the protection of research participants, federal regulations and IRB policy require study teams to submit reportable events to the IRB for review. Reportable events include noncompliance, new information, and potential unanticipated problems.
What are reportable events?
A reportable event is an adverse event or other incident that has the potential to be classified by the IRB as an unanticipated problem posing risks to participants or others.
What is considered a reportable event?
What is a reportable event, and how is it reported? A reportable event is any event that the IRB may determine is an unanticipated problem involving risks to subjects or others or serious or continuing noncompliance with the federal regulations or Institutional Review Board requirements.
What must be reported immediately to the IRB?
Response: Unanticipated adverse device effect. New or increased risk. Protocol deviation that harmed a subject or placed subject at risk of harm. Protocol deviation made without prior IRB approval to eliminate an immediate hazard to a subject. Audit, inspection, or inquiry by a federal agency.
What events are reportable to the IRB?
What Events Must Be Reported to the IRB within 10 Working Days? Non-compliance with the federal regulations governing human research or with the requirements or determinations of the IRB that pose a harm to the rights, safety, or welfare of the subject, or to the integrity of the data.
What types of study events require immediate IRB review in US settings?
Quick Guide: Activities Requiring IRB Review ActivitiesIRB Review Required? Emergency Use of an Investigational Drug or Device YES Classroom Assignments/ Research Methods Classes YES NO (but instructors have an obligation to protect students and others) Research Using Publicly Available Data Sets NO24 more rows • Oct 22, 2024
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Event Requiring Prompt Reporting to the IRB?
An Event Requiring Prompt Reporting to the IRB is a significant occurrence during a research study that may affect the safety, risk, or rights of study participants.
Who is required to file Event Requiring Prompt Reporting to the IRB?
Researchers, principal investigators, and any designated personnel involved in the study are required to file Events Requiring Prompt Reporting to the IRB.
How to fill out Event Requiring Prompt Reporting to the IRB?
To fill out the report, provide detailed information about the event, including date, description, impact on participants, and any corrective actions taken, ensuring all required fields are completed.
What is the purpose of Event Requiring Prompt Reporting to the IRB?
The purpose is to ensure that the IRB is aware of significant changes or incidents that could affect participant safety and to facilitate timely review and response.
What information must be reported on Event Requiring Prompt Reporting to the IRB?
Key information includes the nature of the event, date it occurred, details on affected participants, potential impact on study integrity, and any actions taken to mitigate risks.
Fill out your event requiring prompt reporting online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Event Requiring Prompt Reporting is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















