Get the free Participant Data Sheet - bu
Show details
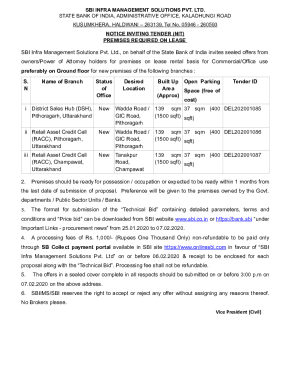
A form for participants in the Public Interest Project that gathers personal and organizational information, and acknowledges understanding of payroll policies and work limitations.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign participant data sheet

Edit your participant data sheet form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your participant data sheet form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing participant data sheet online
Use the instructions below to start using our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit participant data sheet. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
With pdfFiller, it's always easy to work with documents. Check it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out participant data sheet

How to fill out Participant Data Sheet
01
Obtain the Participant Data Sheet from the designated source.
02
Fill in the participant's full name in the designated field.
03
Include the date of birth of the participant.
04
Provide the contact information, including phone number and email address.
05
Fill in the address details, including street, city, state, and zip code.
06
Indicate any relevant medical information or dietary restrictions.
07
Review the sheet for accuracy and completeness.
08
Sign and date the form as required.
Who needs Participant Data Sheet?
01
Researchers conducting studies requiring participant information.
02
Organizations running programs that involve participants.
03
Event coordinators needing participant data for planning purposes.
Fill
form
: Try Risk Free






People Also Ask about
What should a description of a participant include?
If there is space to do so, you can write a brief background of each participant in the “Participants” section and include relevant information on the participant's birthplace, current place of residence, language, and any life experience that is relevant to the study theme.
What is a PIS document?
Portfolio Investment Scheme (PIS) is a scheme of the Reserve Bank of India under which NRIs can purchase or sell shares of listed Indian companies. NRIs can get the PIS letter with the help of the bank where the NRE or NRO account was opened. RBI has authorised only designated branches of a bank to administer the PIS.
What is the purpose of the patient consent form?
The consent form is intended, in part, to provide information for the potential subject's current and future reference and to document the interaction between the subject and the investigator. However, even if a signed consent form is required, it alone does not constitute an adequate consent process.
Why are participant information sheets important?
Information sheets are typically augmented by conversations with research or clinical staff at the point of consent. However, the information sheet is what the potential participant can take away from this encounter and if they do not understand it then there are clearly ethical implications.
What is a PIS form?
Participant Information Sheets and Consent Forms are important aspects to the organisation and conduct of a study.
How do you write a participant information sheet?
Remember who is going to be reading your Participant Information Sheet (PIS). Use short, familiar words and short sentences. Write in simple, non-technical terms that a lay person will easily understand. The language used should be no more difficult to read than information leaflets for medicines or tabloid newspapers.
What is a PIS used for?
A pharmacy information system (PIS) is a software system that records, manages, and stores patient data. This data is then used for patient tracking, decision support, re-ordering, quality assurance, reporting, billing, and ultimately workflow management.
What is a Pis in a clinical trial?
A standard model of the Patient Information Sheet (PIS) and Informed Consent (IC) would facilitate compliance with the guaranteed rights of the patient when their health data is used in any form for purposes other than medical assistance, like the release of case reports and case series.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Participant Data Sheet?
A Participant Data Sheet is a document used to collect and organize essential information about individuals involved in a research study, program, or project.
Who is required to file Participant Data Sheet?
Typically, researchers, project coordinators, or organizations conducting studies involving human participants are required to file a Participant Data Sheet.
How to fill out Participant Data Sheet?
The Participant Data Sheet should be filled out by providing accurate and complete information about each participant, including personal details, consent forms, and any other required data as outlined by the study guidelines.
What is the purpose of Participant Data Sheet?
The purpose of the Participant Data Sheet is to ensure the collection of necessary participant information, facilitate data tracking, and maintain compliance with ethical and legal standards in research.
What information must be reported on Participant Data Sheet?
The information that must be reported on a Participant Data Sheet typically includes participant name, contact information, demographics, medical history, consent status, and any other relevant data as specified by the research protocol.
Fill out your participant data sheet online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Participant Data Sheet is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.