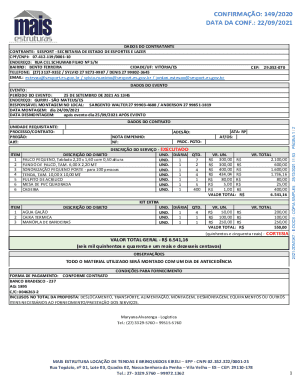
Get the free Principal Investigator Delegation of Responsibilities and Signature Log - mssm
Show details
This document is used to identify and delegate responsibilities among site personnel involved in a clinical trial, ensuring that roles are clearly defined and acknowledged by signatures.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign principal investigator delegation of

Edit your principal investigator delegation of form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your principal investigator delegation of form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing principal investigator delegation of online
Use the instructions below to start using our professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit principal investigator delegation of. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out principal investigator delegation of

How to fill out Principal Investigator Delegation of Responsibilities and Signature Log
01
Begin by gathering all necessary information about the Principal Investigator (PI) and the study.
02
Clearly write the full name and contact information of the PI at the top of the log.
03
List each delegated responsibility along with the corresponding individual's name who will take on that responsibility.
04
Ensure each delegate understands their assigned responsibilities and has agreed to them.
05
Include the signature of the PI to confirm delegation of responsibilities.
06
Obtain signatures from each individual who is delegated responsibilities, indicating their acceptance of the tasks.
07
Review the log for completeness and accuracy before submission.
08
Submit the completed log to the appropriate institutional office for records.
Who needs Principal Investigator Delegation of Responsibilities and Signature Log?
01
Research teams involved in clinical trials or research studies.
02
Institutions requiring formal documentation of accountability and responsibilities.
03
Funding agencies that need to verify delegation of responsibilities by the Principal Investigator.
Fill
form
: Try Risk Free






People Also Ask about
What is the difference between a principal investigator and an investigator?
MIT policy defines Investigators as those individuals who are independently responsible for the design, conduct and reporting of research. The Principal Investigator (PI) is ultimately responsible for all aspects of the project.
What are the responsibilities of a Principal Investigator?
The Principal Investigator (PI) is ultimately responsible for assuring compliance with applicable University IRB policies and procedures, DHHS Federal Policy Regulations, and FDA regulations and for the oversight of the research study and the informed consent process.
What is the primary responsibility of an investigator?
Investigators are ultimately responsible for the conduct of research. Investigators may delegate tasks to appropriately trained and qualified members of their research team. However, investigators must maintain oversight and retain ultimate responsibility for the conduct of those to whom they delegate responsibilities.
What is Delegation of responsibilities in clinical trials?
By definition, delegation of duties is entrusting someone else to do parts of your job. In clinical research, this means investigators can delegate study-related tasks to their staff members to perform on their behalf, but they never relinquish responsibility for those tasks and their outcomes.
What is the job description of a PI?
Conducting surveillance, with the same authority as a private citizen rather than a law enforcement officer, to supplement the information they gather from research and interviews. Tracking suspects and witnesses, watching their homes or places of work while taking photos or video recordings to be used as evidence.
What is the Delegation of responsibilities log?
Details: This log should provide a comprehensive list of study staff members and the duties that have been delegated to them by the Principal Investigator. It is required for both observational and interventional clinical research studies.
What is the main responsibility of a principal investigator?
The Principal Investigator is responsible for the management and integrity of the design, conduct, and reporting of the research project and for managing, monitoring, and ensuring the integrity of any collaborative relationships.
What is the start date on the Delegation log?
The Start Date refers to the date that the individual has been delegated the task by the PI. The individual must be added to the protocol and approved by the IRB and they must receive training for the task to which they have been delegated and this should be documented in a training log.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Principal Investigator Delegation of Responsibilities and Signature Log?
The Principal Investigator Delegation of Responsibilities and Signature Log is a formal document used to record the delegation of specific responsibilities from the principal investigator (PI) to other qualified individuals involved in a research project. It provides a way to track who is responsible for various aspects of the study.
Who is required to file Principal Investigator Delegation of Responsibilities and Signature Log?
The principal investigator and any research personnel who are delegated responsibilities that are outlined in the log are required to file and maintain the Principal Investigator Delegation of Responsibilities and Signature Log.
How to fill out Principal Investigator Delegation of Responsibilities and Signature Log?
To fill out the log, the principal investigator should list the names and roles of individuals to whom specific responsibilities are being delegated, specify the tasks delegated, and include both the PI's and the delegates' signatures along with the date of delegation.
What is the purpose of Principal Investigator Delegation of Responsibilities and Signature Log?
The purpose of the log is to ensure accountability and clarity in the research process by formally documenting who is responsible for different aspects of a study. It also aids in compliance with regulatory and institutional requirements.
What information must be reported on Principal Investigator Delegation of Responsibilities and Signature Log?
The log must report the names of the principal investigator and the individuals to whom responsibilities are delegated, their respective roles, the specific tasks assigned, the dates of delegation, and signatures of both the principal investigator and the delegates.
Fill out your principal investigator delegation of online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Principal Investigator Delegation Of is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.