Get the free CERTIFICATE OF ANALYSIS - umassmed
Show details
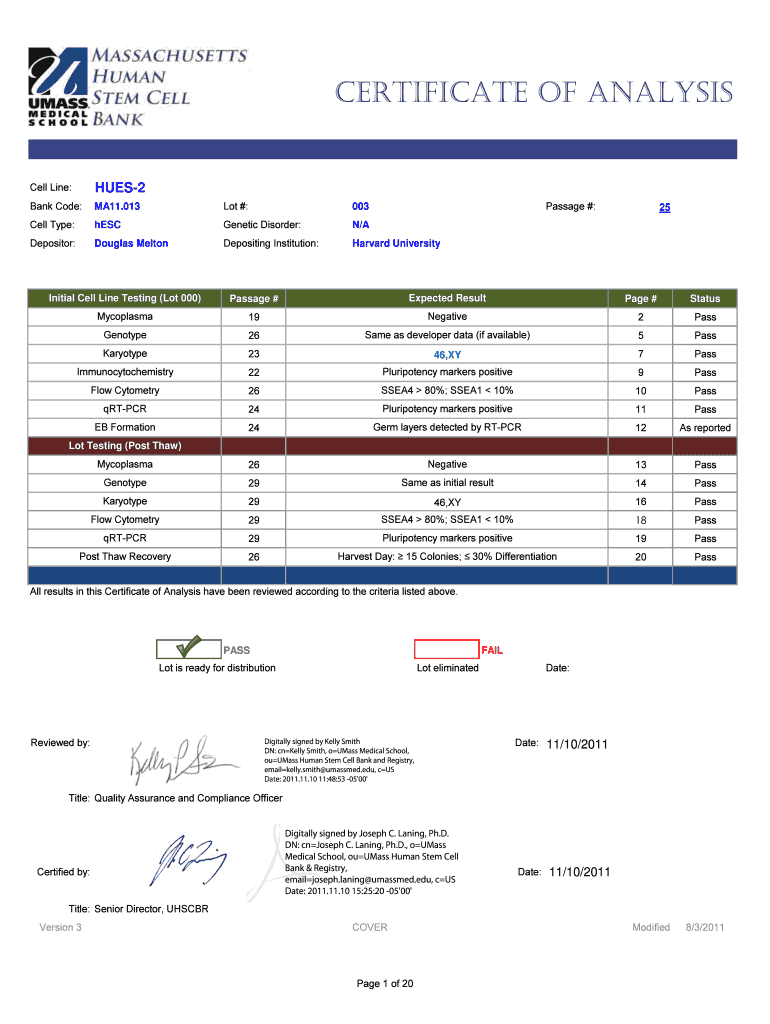
This document provides a comprehensive analysis of the HUES-2 human embryonic stem cell line, including testing results for mycoplasma, genotype, karyotype, immunocytochemistry, flow cytometry, qRT-PCR,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign certificate of analysis

Edit your certificate of analysis form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your certificate of analysis form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing certificate of analysis online
Use the instructions below to start using our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit certificate of analysis. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out certificate of analysis

How to fill out CERTIFICATE OF ANALYSIS
01
Start with the title 'Certificate of Analysis'.
02
Include the name and address of the manufacturer.
03
Specify the product name and batch/lot number.
04
List the relevant tests conducted (e.g., purity, potency, heavy metals).
05
Provide the test method used for each analysis.
06
Present the results for each test, including acceptable limits.
07
State the date of testing and the signature of the responsible person.
08
Add additional remarks if necessary.
Who needs CERTIFICATE OF ANALYSIS?
01
Manufacturers who produce products requiring quality assurance.
02
Distributors who require proof of product compliance.
03
Regulatory bodies that oversee product safety.
04
Customers who need verification of product quality.
Fill
form
: Try Risk Free






People Also Ask about
Is a COA a legal document?
A quick definition of COA: A Certificate of Appealability is a legal document that allows a person to appeal a court decision to a higher court.
Where to get COA certification?
Who Issues the Certificate? The CoA is issued by the Environmental Health Department of the local municipality.
Is a certificate of analysis a legal document?
A Certificate of Analysis (CoA) is a contractual document most often issued by a quality control department. It serves to confirm with evidence (analysis results) that a product is tested ing to certain standards and methods, and that test results are available.
How do you get a certificate of analysis?
A COA is usually issued by a manufacturer's quality assurance or quality control personnel who will then ensure the products' authenticity and that they are fulfilling the prescribed standards set. In some cases, the issuing authority can also come from an inspection agency known by the manufacturer.
Can I get a COA online?
How can I obtain a Certificate of Analysis (COA)? COAs are available on our website. In order to locate the correct COA for any product, enter the lot number that is printed on the product packaging. If you cannot locate your lot number or have any questions, contact Customer Service.
Where can I get a Certificate of Analysis?
A COA is usually issued by a manufacturer's quality assurance or quality control personnel who will then ensure the products' authenticity and that they are fulfilling the prescribed standards set. In some cases, the issuing authority can also come from an inspection agency known by the manufacturer.
How do you write a Certificate of Analysis?
Certificate of Analysis Requirements The name and logo of the company. The product that is being certified. The lot number assigned by the manufacturer. The name of the customer set to receive the batch. All the tests performed on the product. Property of the product, which may include size, color and components.
What is the difference between COA and COC?
A COC assures adherence to regulatory and safety norms, while a COA, often issued by external laboratories, offers detailed test results and analysis, underscoring the product's quality and safety.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is CERTIFICATE OF ANALYSIS?
A Certificate of Analysis (CoA) is a document issued by quality control that confirms that a product meets its specifications and requirements. It typically includes details about the product's test results and compliance with standards.
Who is required to file CERTIFICATE OF ANALYSIS?
Manufacturers, suppliers, and importers of products that require verified quality assurance are typically required to file a Certificate of Analysis to ensure that their products meet regulatory and safety standards.
How to fill out CERTIFICATE OF ANALYSIS?
To fill out a Certificate of Analysis, one must include the product name, batch number, manufacturing date, various test results, and any applicable standards. The document should be signed by an authorized quality control official.
What is the purpose of CERTIFICATE OF ANALYSIS?
The purpose of a Certificate of Analysis is to provide consumers, regulators, and other stakeholders with proof that a product has been tested and meets specified criteria for quality and safety.
What information must be reported on CERTIFICATE OF ANALYSIS?
The information that must be reported on a Certificate of Analysis includes product identification, batch or lot number, testing methods, results of the tests conducted, expiration date, and the signature of the certifying authority.
Fill out your certificate of analysis online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Certificate Of Analysis is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















