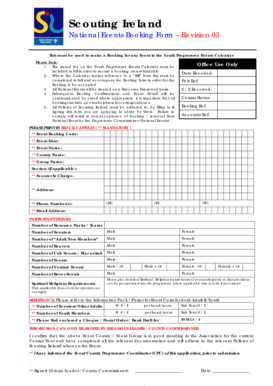
Get the free Vaccine Storage Incident Report
Show details
Este formulario se utiliza cuando se desperdicia una vacuna debido a un fallo de almacenamiento o de energía. Proporciona instrucciones para corregir la situación, registrar temperaturas, contactar
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign vaccine storage incident report

Edit your vaccine storage incident report form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your vaccine storage incident report form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit vaccine storage incident report online
In order to make advantage of the professional PDF editor, follow these steps below:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit vaccine storage incident report. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out vaccine storage incident report

How to fill out Vaccine Storage Incident Report
01
Begin by gathering all necessary information regarding the incident including date, time, and location.
02
Identify the type of vaccine that was affected.
03
Document the reasons for the incident (e.g., power outage, equipment failure).
04
Record the temperature range during the incident, if applicable.
05
Indicate the duration of the storage incident.
06
List the actions taken to address the incident (e.g., notifications, corrective measures).
07
Include details about the affected doses, such as batch numbers and expiration dates.
08
Finally, review the report for accuracy and submit it to the designated authority.
Who needs Vaccine Storage Incident Report?
01
Healthcare providers who store vaccines.
02
Pharmacies administering vaccines.
03
Public health officials responsible for vaccine distribution.
04
Regulatory bodies overseeing vaccine storage compliance.
Fill
form
: Try Risk Free






People Also Ask about
Under what conditions are vaccines stored?
All vaccines must be stored within the +2°C to +8°C temperature range at all times during storage or transport, from the point of manufacture through to the point they are administered to an individual. These standards may also be used to support cold chain management for the storage of other refrigerated medicines. 3.
What are the rules for vaccination storage?
Most vaccines (all inactivated vaccines and live nasal spray influenza vaccine) must be stored between 2° to 8°C (36° to 46°F), which is the recommended refrigerator temperature. Live varicella (chickenpox) and Zostavax (shingles) vaccines must be stored frozen between -50° to -15°C (-58° to +5°F).
What are the storage conditions for official vaccines?
Storage conditions refer to the physical and chemical environmental conditions necessary to preserve the freshness, quality, and safety of stored products. Factors such as the type of product, storage duration, and target market are considered when determining storage conditions.
What are the storage requirements for vaccines?
All vaccines must be stored within the recommended temperature range of +2°C to +8°C at all times. Maintaining the cold chain is important to ensure that effective and potent vaccines are administered to patients.
What is the protocol for vaccine storage?
Temperature Ranges Refrigerators should maintain temperatures between 2° C and 8° C (36° F and 46° F)*. Freezers should maintain temperatures between -50° C and -15° C (-58° F and +5° F). Ultra-cold freezers should maintain temperatures between -90° C and -60° C (-130° F and -76° F) .
What are the reported issues with the COVID vaccine?
To date, the systems in place to monitor the safety of COVID-19 vaccines currently used in the United States have identified anaphylaxis and myocarditis or pericarditis as serious types of adverse events following COVID-19 vaccination.
What is the protocol for storing vaccines?
Temperature Ranges Refrigerators should maintain temperatures between 2° C and 8° C (36° F and 46° F)*. Freezers should maintain temperatures between -50° C and -15° C (-58° F and +5° F). Ultra-cold freezers should maintain temperatures between -90° C and -60° C (-130° F and -76° F) .
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Vaccine Storage Incident Report?
A Vaccine Storage Incident Report is a documentation tool used to record any incidents affecting the storage conditions of vaccines, including temperature deviations, equipment failures, or any related issues that could compromise vaccine integrity.
Who is required to file Vaccine Storage Incident Report?
Any healthcare facility or provider that stores vaccines is required to file a Vaccine Storage Incident Report when an incident occurs that affects the storage conditions.
How to fill out Vaccine Storage Incident Report?
To fill out the Vaccine Storage Incident Report, one should provide details about the incident, including the date and time of occurrence, the type of vaccine affected, the nature of the incident, actions taken to resolve the issue, and any other relevant information.
What is the purpose of Vaccine Storage Incident Report?
The purpose of the Vaccine Storage Incident Report is to ensure accountability, provide a clear record of the incident for future reference, and to assess the impact on vaccine viability, thereby facilitating proper follow-up actions.
What information must be reported on Vaccine Storage Incident Report?
The report must include the incident date and time, specific vaccines involved, storage conditions before and after the incident, corrective actions taken, potential impact on vaccine efficacy, and any notifications made to relevant stakeholders.
Fill out your vaccine storage incident report online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Vaccine Storage Incident Report is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.