Get the free Anexo 2.12 - repositorium sdum uminho
Show details
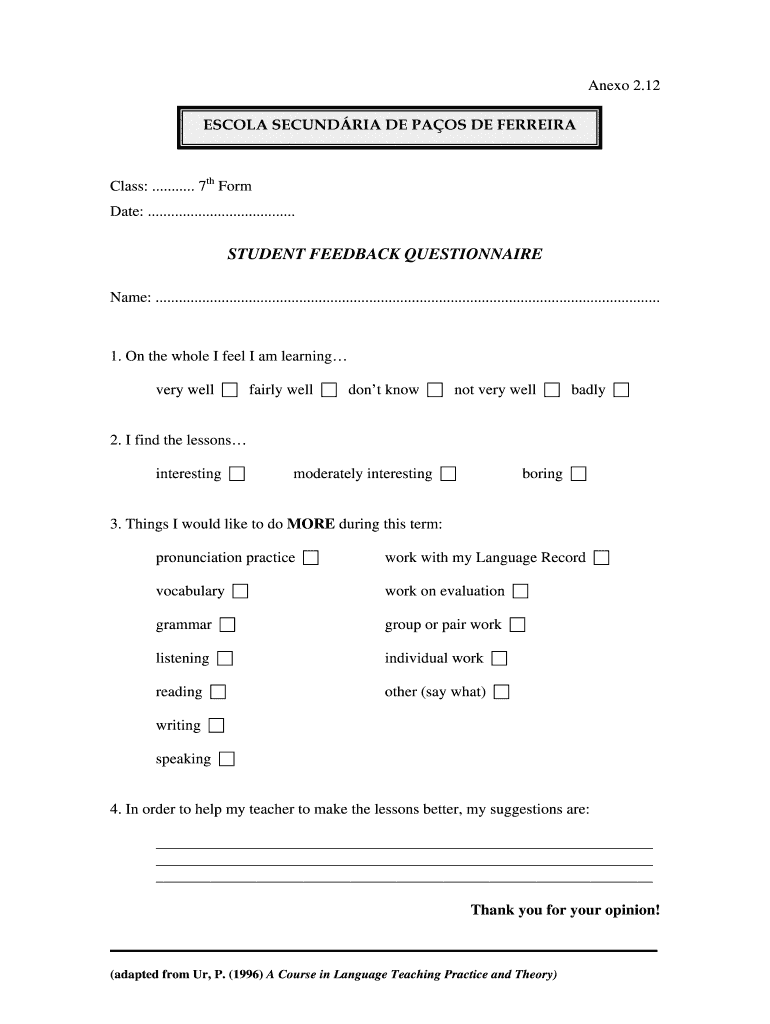
This document is a feedback questionnaire for students to express their learning experiences and suggestions for improving lessons.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign anexo 212 - repositorium

Edit your anexo 212 - repositorium form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your anexo 212 - repositorium form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing anexo 212 - repositorium online
To use the services of a skilled PDF editor, follow these steps below:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit anexo 212 - repositorium. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
It's easier to work with documents with pdfFiller than you can have believed. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out anexo 212 - repositorium

How to fill out Anexo 2.12
01
Obtain the Anexo 2.12 form from the official website or the appropriate government office.
02
Begin filling out the personal identification section with your full name, address, and contact information.
03
Provide any required identification numbers, such as your tax ID or social security number.
04
Fill in the relevant financial information as requested in the form, including income sources and expenses.
05
Review any guidelines or specific instructions included with the form to ensure compliance.
06
Double-check all the information for accuracy and completeness before submitting.
07
Submit the completed form to the designated office or through the online portal if applicable.
Who needs Anexo 2.12?
01
Individuals or businesses applying for certain government benefits or tax considerations.
02
Tax professionals and accountants preparing financial documentation for their clients.
03
Entities that need to report specific financial information as part of regulatory compliance.
Fill
form
: Try Risk Free






People Also Ask about
What is the current medical device directive?
Medical device legislation The Medical Devices Regulation applies since 26 May 2021. Manufacturers must comply with the Regulation when placing new medical devices on the market. It repeals Directive 93/42/EEC on medical devices and the Directive 90/385/EEC on active implantable medical devices.
Are MEDDEV documents still valid?
With the replacement of the EU directives MDD, AIMDD, and IVDD by the EU regulations MDR and IVDR, the MEDDEV documents are largely obsolete. One exception is, for example, the MEDDEV 2.7/1 rev 4 guideline. Manufacturers and notified bodies should, therefore, always check whether there is a suitable MDCG guideline.
What is the current revision of MEDDEV 2.7 1?
Revision 4 of Clinical Evaluation guidance document MEDDEV 2.7. 1 was released by the European Commission on 1 July 2016. A copy of the latest revision can be downloaded from our website.
Is Meddev 2.12 1 still valid?
MEDDEV 2.12-1 Rev 8 provides guidance to medical device manufacturers on market surveillance. It continues to be the primary guidance document for vigilance reporting, even with the implementation of the new EU MDR and IVDR.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Anexo 2.12?
Anexo 2.12 is a specific form used in certain tax jurisdictions for reporting various financial or operational information as part of the annual tax filings.
Who is required to file Anexo 2.12?
Taxpayers, including individuals and businesses, who meet specific criteria set by the tax authority in their jurisdiction, are required to file Anexo 2.12.
How to fill out Anexo 2.12?
To fill out Anexo 2.12, taxpayers must gather the required documentation, complete all sections of the form accurately, and ensure all information is in compliance with the relevant tax regulations.
What is the purpose of Anexo 2.12?
The purpose of Anexo 2.12 is to provide the tax authorities with detailed information regarding a taxpayer's financial activities, which aids in the assessment of tax obligations.
What information must be reported on Anexo 2.12?
Anexo 2.12 typically requires reporting on income, expenses, assets, and liabilities, as well as any other relevant financial data specific to the taxpayer's situation.
Fill out your anexo 212 - repositorium online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Anexo 212 - Repositorium is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















