Get the free MSH REB EXTERNAL SERIOUS ADVERSE EVENT SUMMARY REPORTING FORM - mountsinai on
Show details
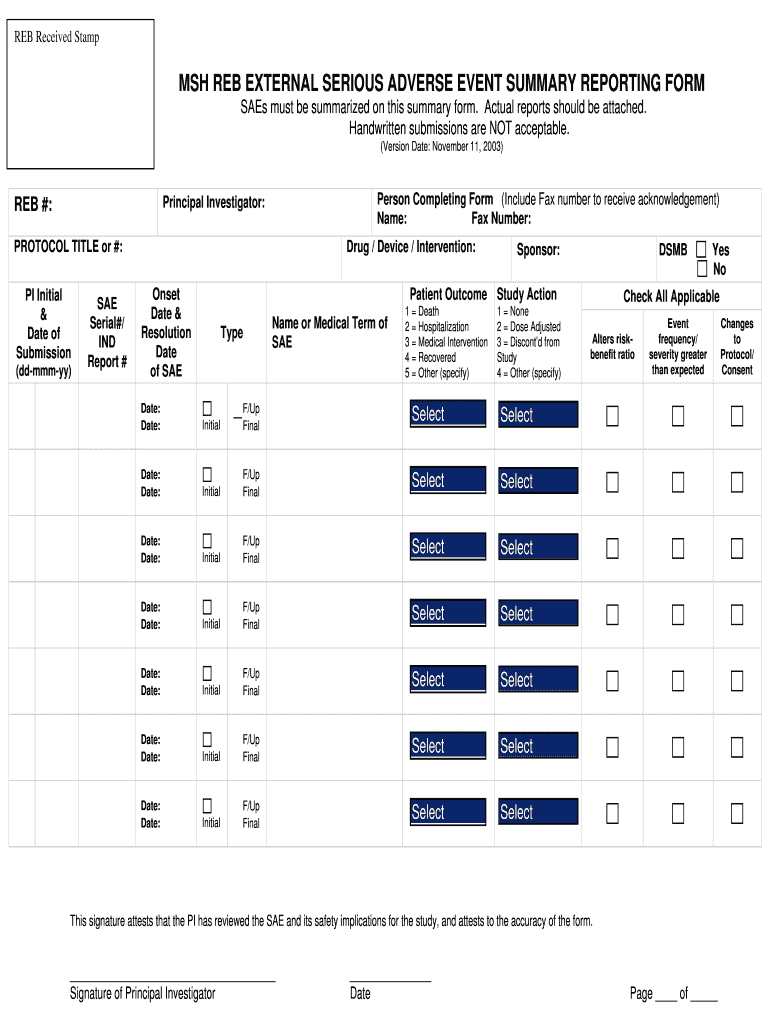
This form is used to summarize serious adverse events (SAEs) associated with a research study. It requires detailed information about the event, patient outcomes, and actions taken during the study.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign msh reb external serious

Edit your msh reb external serious form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your msh reb external serious form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit msh reb external serious online
Use the instructions below to start using our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit msh reb external serious. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out msh reb external serious

How to fill out MSH REB EXTERNAL SERIOUS ADVERSE EVENT SUMMARY REPORTING FORM
01
Begin with the form title and ensure it is the MSH REB EXTERNAL SERIOUS ADVERSE EVENT SUMMARY REPORTING FORM.
02
Fill in the basic information section, including the study title, protocol number, and report date.
03
Enter participant information, ensuring to include participant ID and demographics if necessary.
04
In the adverse event description section, provide a detailed account of the event, including onset date, duration, and outcomes.
05
Specify the relationship of the adverse event to the study intervention, marking whether it is related, possibly related, or unlikely related.
06
Include any actions taken in response to the adverse event, such as modifications to the study or interventions for the participant.
07
Document any follow-up information or additional comments that may be relevant to the adverse event.
08
Review the completed form for accuracy and completeness before submission.
09
Submit the form to the appropriate regulatory body or ethics board as required.
Who needs MSH REB EXTERNAL SERIOUS ADVERSE EVENT SUMMARY REPORTING FORM?
01
Researchers conducting clinical trials or studies that report serious adverse events.
02
Institutional review boards (IRBs) or research ethics boards (REBs) that require documentation of adverse events.
03
Participants in clinical trials who might need to report adverse events to ensure their safety.
Fill
form
: Try Risk Free






People Also Ask about
Do adverse events need to be reported to the IRB?
The MedWatch form, also known as Form FDA 3500A, is used for mandatory reporting of medical device adverse events by manufacturers, user facilities and importers.
What are the requirements for reporting adverse events?
Initial reporting: Any suspected adverse events or any adverse events that are considered serious and unexpected must be reported to the FDA as soon as possible but no later than within 15 calendar days of first being notified of the event.
What are the 4 criteria for adverse event reporting?
MedWatch is the Food and Drug Administration's (FDA) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure, and product use errors with human medical products, including drugs, biologic products, medical devices, dietary supplements, infant formula, and cosmetics.
What are the four main details required to report an adverse event?
Information about the person who had the adverse reaction (such as age and gender); A description of the adverse reaction; The dose and name of the medicinal product suspected of causing the adverse reaction; The batch number of the medicinal product (indicated on the package);
How to report a serious adverse event?
These types of events must be reported to the IRB using the AE form if they are also serious. Any AE that results in any of the following outcomes: Death, Life-threatening adverse experience**
What are the 4 elements of an adverse event?
The minimum dataset required to consider information as a reportable AE is indeed minimal, namely (1) an identifiable patient, (2) an identifiable reporter, (3) product exposure, and (4) an event.
What are the 4 criteria for a valid ICSR?
For reporting purposes, done electronically in EU/EEA, the ICSR should contain the following 4 basic elements: An identifiable patient/subject; An identifiable reporter, A suspect drug or biological product, An adverse event or fatal outcome.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is MSH REB EXTERNAL SERIOUS ADVERSE EVENT SUMMARY REPORTING FORM?
The MSH REB EXTERNAL SERIOUS ADVERSE EVENT SUMMARY REPORTING FORM is a standardized document used to report serious adverse events that occur during clinical research or healthcare interventions. It ensures proper documentation and evaluation of adverse events to maintain participant safety.
Who is required to file MSH REB EXTERNAL SERIOUS ADVERSE EVENT SUMMARY REPORTING FORM?
Investigators, research staff, and healthcare providers involved in clinical trials or studies are typically required to file the MSH REB EXTERNAL SERIOUS ADVERSE EVENT SUMMARY REPORTING FORM whenever a serious adverse event occurs.
How to fill out MSH REB EXTERNAL SERIOUS ADVERSE EVENT SUMMARY REPORTING FORM?
To fill out the form, one must provide detailed information about the adverse event, including participant identification, event description, severity, relationship to the study, and any actions taken. Each section of the form should be completed with accurate and concise information.
What is the purpose of MSH REB EXTERNAL SERIOUS ADVERSE EVENT SUMMARY REPORTING FORM?
The purpose of the form is to systematically collect data on serious adverse events, facilitate timely reporting to regulatory bodies, and enhance the safety monitoring of clinical trials, ultimately improving participant safety and study integrity.
What information must be reported on MSH REB EXTERNAL SERIOUS ADVERSE EVENT SUMMARY REPORTING FORM?
The information required includes the event date, description of the event, participant's medical history, details of the intervention involved, assessment of severity, causality, and any concomitant medications or treatments provided.
Fill out your msh reb external serious online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Msh Reb External Serious is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















