Get the free Evidence in Complementary Medicine Registration Form - ucalgary
Show details
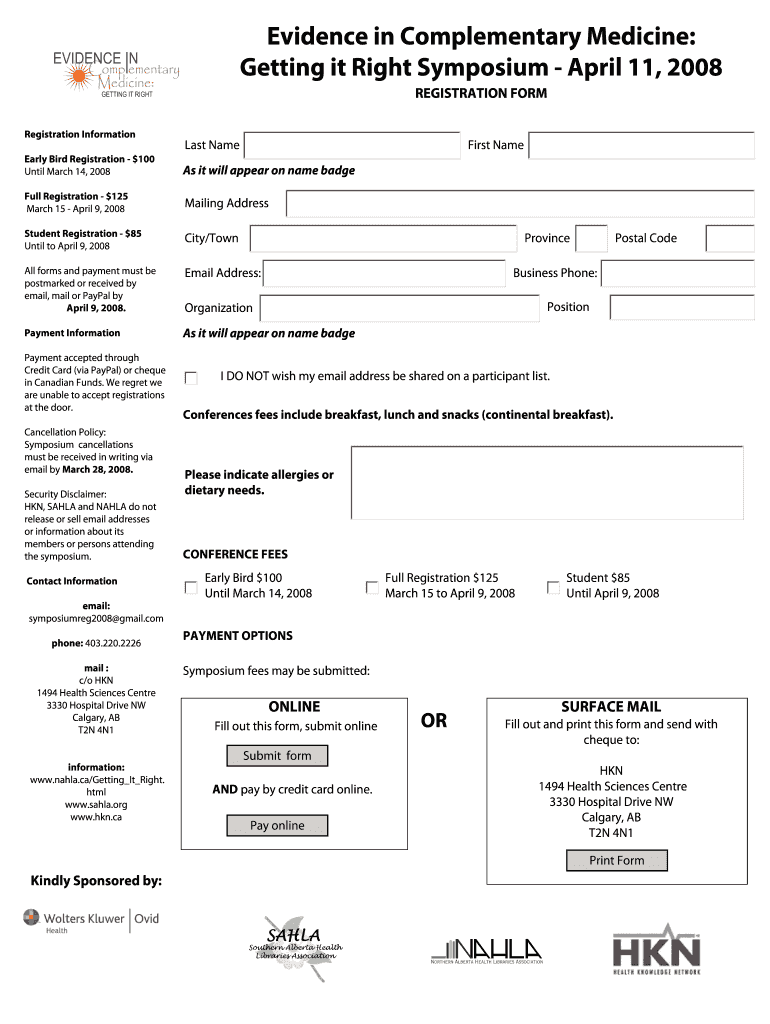
A registration form for attendees of the Evidence in Complementary Medicine symposium, providing details on registration types, payment options, and contact information.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign evidence in complementary medicine

Edit your evidence in complementary medicine form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your evidence in complementary medicine form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing evidence in complementary medicine online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log in to your account. Start Free Trial and register a profile if you don't have one yet.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit evidence in complementary medicine. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out evidence in complementary medicine

How to fill out Evidence in Complementary Medicine Registration Form
01
Obtain the Complementary Medicine Registration Form from the official website or relevant authority.
02
Fill in your personal information including name, address, and contact details in the designated sections.
03
Provide detailed information about your complementary medicine practice, including the modalities you use.
04
Gather evidence supporting your qualifications and experience in complementary medicine, such as certificates, licenses, and training records.
05
Attach copies of any relevant documentation that supports your claim for registration.
06
Complete any additional sections that may require information about your clinical experience or case studies.
07
Review the entire form for accuracy and completeness.
08
Submit the form along with the evidence to the designated authority, either online or by post.
Who needs Evidence in Complementary Medicine Registration Form?
01
Practitioners of complementary medicine seeking to register their practice legally.
02
Individuals applying for certification in complementary medicine modalities.
03
Health and wellness professionals transitioning to complementary medicine practices.
04
Students or recent graduates of complementary medicine programs looking to establish their credentials.
Fill
form
: Try Risk Free






People Also Ask about
How are complementary therapies regulated?
Codes of professional conduct and public accountability Most complementary medicine organisations are run as conventional professional bodies; they publish formal codes of ethics and practice, and registers of their members are available to the public.
Is complementary medicine evidence-based?
Treatment outcomes for diseases with evidence-based complementary and alternative medicine (EBCAM) have been remarkably successful. To share evidence-based information, contemporary CAM systems must be integrated. Today, many patients seek healing through both alternative and conventional medicine.
How is CAM regulated by the FDA?
First, a CAM product might be subject to regulation as a drug, a biological or cosmetic device, or a food (including food additives and dietary supplements) under the Federal Food, Drug, and Cosmetic (FFDC) Act or the Public Health Service (PHS) Act. These statutory classifications cover several CAM products.
What do you need to know about complementary and alternative medicine?
Complementary and alternative medicine includes practices such as massage, acupuncture, tai chi, and drinking green tea. Integrative medicine is an approach to medical care that combines conventional medicine with CAM practices that have shown through science to be safe and effective.
What is the complementary system of medicine?
Complementary and alternative medicine includes practices such as massage, acupuncture, tai chi, and drinking green tea. Integrative medicine is an approach to medical care that combines conventional medicine with CAM practices that have shown through science to be safe and effective.
What is the regulation of complementary medicines?
The regulation of complementary medicines helps to protect the public. It helps ensure that therapeutic goods are produced to an acceptable standard of safety and quality (good manufacturing practice) and that any adverse reactions can be investigated.
What is the Complementary and Alternative Medicine Act?
THE TRADITIONAL AND COMPLEMENTARY MEDICINE ACT, 2019. An Act to define traditional and complementary medicine in relation to modern medicine, to establish a Council to control and regulate the practice of traditional and complementary medicine, to register and license practitioners and to provide for related matters.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Evidence in Complementary Medicine Registration Form?
Evidence in Complementary Medicine Registration Form refers to the documentation and proof required to validate the efficacy, safety, and regulatory compliance of complementary medicine practices or products.
Who is required to file Evidence in Complementary Medicine Registration Form?
Practitioners and businesses offering complementary medicine therapies, products, or services are required to file the Evidence in Complementary Medicine Registration Form to ensure they meet regulatory standards.
How to fill out Evidence in Complementary Medicine Registration Form?
To fill out the Evidence in Complementary Medicine Registration Form, you must provide specific details about your practice or product, including clinical evidence, research support, safety data, and additional information as required by the regulatory body.
What is the purpose of Evidence in Complementary Medicine Registration Form?
The purpose of the Evidence in Complementary Medicine Registration Form is to ensure that complementary medicine practitioners and products are safe, effective, and comply with relevant laws and regulations.
What information must be reported on Evidence in Complementary Medicine Registration Form?
The information that must be reported includes the type of complementary medicine, details of clinical studies or trials, safety assessments, practitioner qualifications, and any adverse effects reported.
Fill out your evidence in complementary medicine online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Evidence In Complementary Medicine is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















