Get the free Common Carp Pituitary Clinical Field Trials CCP-2: Drug Inventory Form - Version 4 -...
Show details
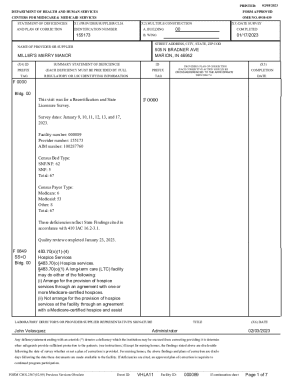
This document serves as a drug inventory and tracking form for the Common Carp Pituitary used in clinical field trials, detailing requirements for recording usage, transfers, and disposals of the
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign common carp pituitary clinical

Edit your common carp pituitary clinical form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your common carp pituitary clinical form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing common carp pituitary clinical online
Follow the guidelines below to use a professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit common carp pituitary clinical. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
With pdfFiller, dealing with documents is always straightforward. Try it right now!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out common carp pituitary clinical

How to fill out Common Carp Pituitary Clinical Field Trials CCP-2: Drug Inventory Form - Version 4
01
Download the Common Carp Pituitary Clinical Field Trials CCP-2: Drug Inventory Form Version 4 from the official website.
02
Review the form instructions carefully before filling it out.
03
Enter the date of the inventory at the top of the form.
04
List each drug name in the designated columns, ensuring accurate spelling and classification.
05
Fill in the quantity of each drug available in the next column.
06
Note the manufacturer and supplier of the drugs for traceability.
07
Include any expiration dates associated with the drugs.
08
Indicate the storage conditions necessary for each drug.
09
Provide any additional comments in the comments section if applicable.
10
Review all filled sections for accuracy before submission.
11
Submit the completed form to the designated authority or department.
Who needs Common Carp Pituitary Clinical Field Trials CCP-2: Drug Inventory Form - Version 4?
01
Researchers involved in Common Carp Pituitary Clinical Field Trials who require accurate drug inventory management.
02
Regulatory bodies managing clinical trials for compliance and oversight.
03
Pharmaceutical companies supplying drugs for the trials.
04
Clinical trial administrators overseeing trial logistics.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Common Carp Pituitary Clinical Field Trials CCP-2: Drug Inventory Form - Version 4?
The Common Carp Pituitary Clinical Field Trials CCP-2: Drug Inventory Form - Version 4 is a standardized document used in clinical trials to track and manage drug inventory specifically for studies involving common carp and their pituitary-related treatments.
Who is required to file Common Carp Pituitary Clinical Field Trials CCP-2: Drug Inventory Form - Version 4?
Researchers and clinical trial coordinators involved in the Common Carp Pituitary Clinical Field Trials are required to file the CCP-2: Drug Inventory Form - Version 4 as part of compliance and documentation for drug usage in the trials.
How to fill out Common Carp Pituitary Clinical Field Trials CCP-2: Drug Inventory Form - Version 4?
To fill out the Common Carp Pituitary Clinical Field Trials CCP-2: Drug Inventory Form - Version 4, users should gather all required data regarding drug quantities, storage conditions, administration times, and participant identifiers, then input this information into the designated fields of the form accurately.
What is the purpose of Common Carp Pituitary Clinical Field Trials CCP-2: Drug Inventory Form - Version 4?
The purpose of the Common Carp Pituitary Clinical Field Trials CCP-2: Drug Inventory Form - Version 4 is to provide a systematic approach to documenting drug inventory which ensures accountability, traceability, and adherence to trial protocols.
What information must be reported on Common Carp Pituitary Clinical Field Trials CCP-2: Drug Inventory Form - Version 4?
The information that must be reported includes drug name, batch number, quantity received, quantity used, storage location, expiration dates, and details of the individuals involved in handling the drugs throughout the clinical trial process.
Fill out your common carp pituitary clinical online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Common Carp Pituitary Clinical is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.