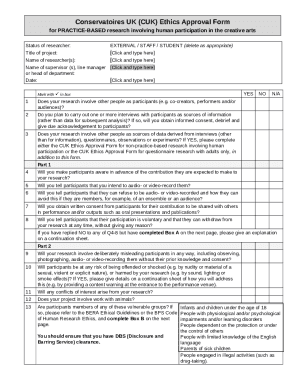
Get the free Responsibilities in Medical Device Clinical Trials
Show details
This document outlines the responsibilities of sponsors in medical device clinical trials, highlighting regulatory definitions, key sponsor duties, monitoring roles, and record-keeping requirements,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign responsibilities in medical device

Edit your responsibilities in medical device form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your responsibilities in medical device form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing responsibilities in medical device online
To use our professional PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit responsibilities in medical device. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
With pdfFiller, it's always easy to work with documents. Try it!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out responsibilities in medical device

How to fill out Responsibilities in Medical Device Clinical Trials
01
Review the clinical trial protocol to understand the objectives and scope.
02
Identify the specific roles within the clinical trial team, such as Principal Investigator, Clinical Research Coordinator, and Regulatory Affairs Specialist.
03
List the tasks associated with each role, ensuring that each responsibility is clear and actionable.
04
Include responsibilities related to patient recruitment, data collection, and reporting.
05
Specify regulatory compliance requirements and responsibilities related to safety monitoring.
06
Ensure that responsibilities are aligned with the necessary training and qualifications of the team members.
07
Document communication protocols among team members regarding responsibilities.
08
Review and revise the responsibilities section as needed based on feedback and changes in the trial.
Who needs Responsibilities in Medical Device Clinical Trials?
01
Clinical trial sponsors who are overseeing the study.
02
Regulatory authorities who require adherence to guidelines.
03
Clinical research teams that need clarity on their roles.
04
Institutional Review Boards (IRBs) for ethical oversight.
05
Site coordinators managing the logistics of the trial.
Fill
form
: Try Risk Free






People Also Ask about
What are the roles and responsibilities of clinical trial personnel?
Key Responsibilities: Assisting with informed consent processes. Coordinating data collection and monitoring patient progress. Ensuring proper documentation and record-keeping. Managing patient safety and reporting adverse events.
What is the difference between QA and QC in clinical trials?
QC, or Quality Control, monitors and verifies data, processes, and compliance with established standards. In contrast, QA, or Quality Assurance, encompasses the development of robust quality policies, risk management, and adherence to standard operating procedures.
What is the role of QA in clinical trials?
The QA team performs on-site evaluations during the trial to ensure compliance with protocols and regulations, address safety and well-being, and verify the resolution of issues reported by trial monitors.
How much does a clinical specialist make in the US?
The average salary for a Clinical Specialist is $160,962 per year or $77 per hour in United States, which is in line with the national average. Top earners have reported making up to $264,989 (90th percentile).
What does a medical device clinical specialist do?
As a medical device clinical specialist, you sell medical devices and equipment to healthcare service providers, medical research specialists, and hospitals. Your duties and responsibilities include traveling to trade shows and medical conferences, or to medical facilities.
What is the role of QA testing?
Quality assurance (QA) testers play a critical role in delivering high quality, perfectly-functioning software and web applications to customers. They test and evaluate new and existing programs to identify and help remove bugs, glitches, and other user experience issues.
What is the role of quality assurance in clinical trials?
Clinical trial quality assurance is a critical element of clinical research, as it ensures the safety of the participants, as well as the reliability and integrity of the documentation and data. By providing assurance the trial adheres to quality standards, it therefore assures the validity of your results.
Which tasks are clinical trial responsibilities of the investigator?
Investigators are responsible for supervising the proper handling, administration, storage, and destruction of investigational agents (ie, drug accountability). Although these tasks can be delegated to an appropriately qualified individual, the investigator maintains ultimate responsibility.
What is the role of a clinical specialist?
What Does a Clinical Specialist Do? As a clinical specialist, you work in a medical laboratory. Your job is to help test samples or evaluate the potential benefits of a medical product. Most people in this field specialize in a specific type of test or focus on a specific medical condition.
What is the role of a medical device clinical specialist?
As a medical device clinical specialist, you sell medical devices and equipment to healthcare service providers, medical research specialists, and hospitals. Your duties and responsibilities include traveling to trade shows and medical conferences, or to medical facilities.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Responsibilities in Medical Device Clinical Trials?
Responsibilities in Medical Device Clinical Trials refer to the specific roles, obligations, and duties assigned to various stakeholders, including sponsors, investigators, and regulatory bodies, throughout the clinical trial process.
Who is required to file Responsibilities in Medical Device Clinical Trials?
Typically, the sponsor of the clinical trial, which may be a company or organization conducting the study, is required to file the Responsibilities. Investigators and other key personnel involved in the trial may also need to report their specific responsibilities.
How to fill out Responsibilities in Medical Device Clinical Trials?
To fill out Responsibilities in Medical Device Clinical Trials, stakeholders should provide detailed descriptions of each person's role, ensure compliance with regulatory guidelines, and document any commitments, including data collection, patient safety monitoring, and reporting procedures.
What is the purpose of Responsibilities in Medical Device Clinical Trials?
The purpose of Responsibilities in Medical Device Clinical Trials is to clearly define each party's obligations to ensure the trial is conducted ethically, safely, and in compliance with regulatory standards, thus contributing to the integrity of the study results.
What information must be reported on Responsibilities in Medical Device Clinical Trials?
Information that must be reported includes the specific roles and responsibilities of each participant in the trial, processes for monitoring patient safety, data management procedures, and reporting obligations to regulatory authorities.
Fill out your responsibilities in medical device online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Responsibilities In Medical Device is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.