Get the free CLINICAL REVIEW
Show details
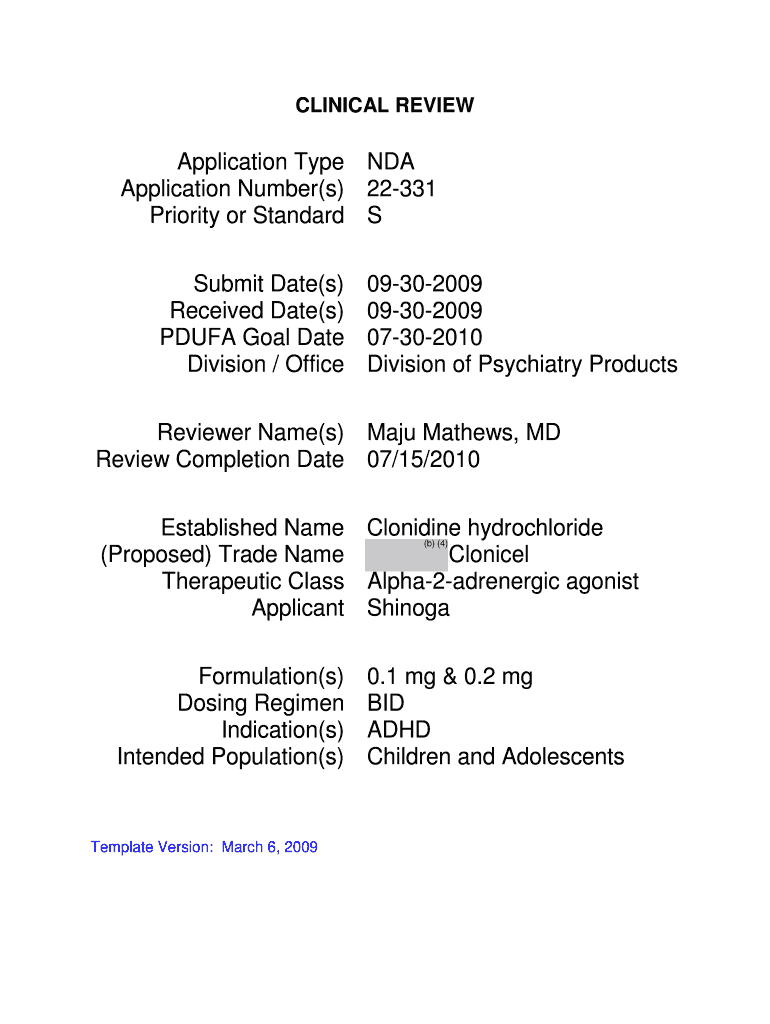
This document provides a comprehensive clinical review of the NDA application for Clonicel, an alpha-2-adrenergic agonist, focusing on its efficacy and safety for treating Attention Deficit Hyperactivity
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical review

Edit your clinical review form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical review form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing clinical review online
Follow the guidelines below to benefit from a competent PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit clinical review. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
Dealing with documents is always simple with pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical review

How to fill out CLINICAL REVIEW
01
Read the clinical review guidelines provided by your institution.
02
Gather all relevant patient data, including medical history and current medications.
03
Review the patient's symptoms and diagnosis thoroughly.
04
Note any prior clinical reviews or treatments administered.
05
Document the findings in a structured format, identifying key areas of concern.
06
Provide evidence-based recommendations and potential treatment options.
07
Ensure all information is accurate and up-to-date.
08
Submit the review to the appropriate healthcare professional for evaluation.
Who needs CLINICAL REVIEW?
01
Medical professionals conducting assessments for patient care.
02
Patients undergoing evaluations for treatment plans.
03
Healthcare organizations requiring clinical data for quality assurance.
04
Research teams needing comprehensive patient evaluations.
Fill
form
: Try Risk Free






People Also Ask about
How do you write a clinical review?
Elements of a review article Title page: Length: The title should be in a range of 8 -12 words. describing the topic and its aspect. Abstract: Citation: No citations needed in the abstract. Introduction: Tense: Should be written in present tense addressing the research question. Materials and Methods.
What happens in a clinical review?
A Full Clinical Review is a comprehensive evaluation of a patient's health. It thoroughly assesses medical history, current symptoms, medications, and lifestyle factors. The review may include physical examinations, diagnostic tests, and consultations with various healthcare professionals.
What is the meaning of clinical review?
A recommended clinical review is a review of a proposed treatment by your insurer's medical staff. During this review, the experts evaluating the clinical recommendation will decide if they agree that the treatment is right for your health needs. Recommended clinical reviews are done before you get care.
What is a clinical review?
A summary of the clinical literature. A systematic review is a critical assessment and evaluation of all research studies that address a particular clinical issue. The researchers use an organized method of locating, assembling, and evaluating a body of literature on a particular topic using a set of specific criteria.
What are the goals of a clinical review?
The clinical review's main objectives are: Analyze the effectiveness of drug treatment and the safety of the recommended course of action. Analyze the disease's progression, the need for any therapy changes, the need for monitoring, if any, and analyze how convenient therapy is (to improve compliance)
What is clinical literature review?
Traditional clinical review articles, also known as updates, differ from systematic reviews and meta-analyses. Systematic reviews comprehensively examine the medical literature, seeking to identify and synthesize all relevant information to formulate the best approach to diagnosis or treatment.
What does a clinical reviewer do?
It involves the systematic evaluation of clinical practices, medical records, and patient care procedures to ensure they meet established standards. Clinical review is multifaceted, covering a wide range of elements, from patient outcomes to adherence to clinical guidelines and protocols.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is CLINICAL REVIEW?
CLINICAL REVIEW is a systematic evaluation of clinical data to assess the safety, efficacy, and quality of a medical product, often conducted by regulatory bodies or within clinical research settings.
Who is required to file CLINICAL REVIEW?
Typically, sponsors of clinical trials, which include pharmaceutical companies, biotechnology firms, and academic researchers, are required to file CLINICAL REVIEW with the appropriate regulatory authorities.
How to fill out CLINICAL REVIEW?
Filling out CLINICAL REVIEW generally involves compiling clinical trial data, patient demographics, treatment protocols, results, and statistical analyses into a standardized format as required by regulatory bodies.
What is the purpose of CLINICAL REVIEW?
The purpose of CLINICAL REVIEW is to evaluate and ensure that a medical product is safe and effective for its intended use, and to support regulatory approvals and market authorization.
What information must be reported on CLINICAL REVIEW?
Information reported on CLINICAL REVIEW should include study objectives, methods, patient demographics, treatment outcomes, adverse events, and conclusions drawn from the data.
Fill out your clinical review online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Review is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















