Get the free IRB 1998-34
Show details
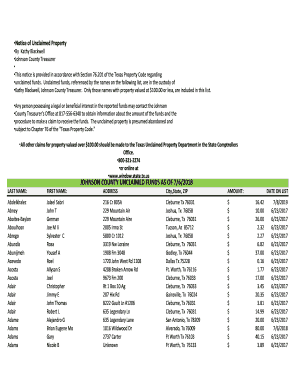
This document outlines the proposed regulations concerning the application of the separate return limitation year (SRLY) rules, addressing net operating loss and capital loss carryovers, and the implications
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign irb 1998-34

Edit your irb 1998-34 form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your irb 1998-34 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit irb 1998-34 online
To use the services of a skilled PDF editor, follow these steps:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit irb 1998-34. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
Dealing with documents is always simple with pdfFiller. Try it right now
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out irb 1998-34

How to fill out IRB 1998-34
01
Obtain a copy of the IRB 1998-34 form from the appropriate regulatory body.
02
Review the guidelines and instructions provided with the form.
03
Fill in the applicant's name and contact information at the top of the form.
04
Provide a detailed description of the research project, including objectives, methodology, and expected outcomes.
05
Indicate the funding source, if applicable.
06
Detail the potential risks and benefits to participants involved in the research.
07
Describe how informed consent will be obtained from participants.
08
Include any necessary supporting documents, such as research proposals, consent forms, and data management plans.
09
Review the completed form for accuracy and completeness.
10
Submit the form to the appropriate IRB for review and approval.
Who needs IRB 1998-34?
01
Researchers conducting studies involving human subjects.
02
Institutional representatives managing research compliance.
03
Students conducting research as part of academic programs.
04
Any individual or organization seeking approval for research projects from an IRB.
Fill
form
: Try Risk Free






People Also Ask about
What are the three types of IRB review?
IRB must review all projects that meet the definition of research and that involve human subjects prior to any data collection to determine the appropriate level of review, and, as appropriate, approve them. There are three major types of review: Exempt, Expedited, and Full.
What are the three levels of risk as outlined by the IRB?
Level of Review and Minimal Risk If your study needs IRB review, the next step is to identify the level of review required – full committee review, expedited review or exempt certification. The level of review reflects the level of risk to the subject.
What are the requirements of an investigator's brochure?
Information in the IB should be presented in a concise, simple, objective, balanced, and non-promotional form that enables a clinician, or potential investigator, to understand it and make his/her own unbiased risk-benefit assessment of the appropriateness of the proposed trial.
What projects do not need IRB approval?
Examples of Studies that Generally Do Not Require IRB Review Data collected for internal departmental or administrative purposes, such as teaching evaluations, student performance data, etc. Activities designed solely for quality improvement or evaluation of a program, course, etc.
Which type of IRB does not require an IRB approval?
Exempt human subjects research is a specific sub-set of “research involving human subjects” that does not require ongoing IRB oversight.
How do I make an IRB amendment?
“Exempt” human subjects research is a sub-set of research involving human subjects that does not require comprehensive IRB review and approval because the only research activity involving the human subjects falls into one or more specific exemption categories as defined by the Common Rule.
What are the 3 key principles for IRB approval?
When reviewing research IRBs are guided by three ethical principles that are fundamental to human subject protection - respect for persons, beneficence, and justice.
Does an investigator brochure need an IRB approval?
To submit a change to your approved IRB protocol: Submit an amendment form explaining the changes being made. Submit an updated application with the changes underlined. Submit any other documents that require updating due to the changes being made (informed consent(s), recruitment materials, etc.).
What are the different types of IRB review?
There are five types of IRB review: (a) exempt, (b) expedited, (c) full, (d) continuing, and (e) limited. An exempt review doesn't require monitoring by the IRB. Exempt categories are outlined by the Department of Health and Human Services in 45 CFR 46.101(b).
Should investigator brochure be reviewed?
The IB shall be updated when new and relevant safety information becomes available, and shall be reviewed by the sponsor at least once per year (CTR article 55.2).
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is IRB 1998-34?
IRB 1998-34 is a regulatory guidance issued by the Internal Revenue Service (IRS) regarding certain tax-related matters.
Who is required to file IRB 1998-34?
Entities and individuals who are involved in tax-exempt organizations or certain transactions that are covered under the guidance of IRB 1998-34 are required to file it.
How to fill out IRB 1998-34?
To fill out IRB 1998-34, one must follow the specific instructions provided in the guidance, which typically includes providing required financial and operational information.
What is the purpose of IRB 1998-34?
The purpose of IRB 1998-34 is to offer clarification and guidance on compliance with tax laws for tax-exempt organizations.
What information must be reported on IRB 1998-34?
The information that must be reported includes details on financial activity, organizational structure, compliance with tax laws, and any relevant transactions.
Fill out your irb 1998-34 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Irb 1998-34 is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.