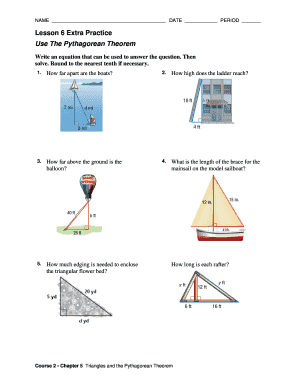
Get the free Guidelines for Submitting Specimens for Influenza 2011-2012 - cdph ca
Show details
This document provides guidelines for healthcare providers in California on how to properly collect and submit specimens for influenza testing during the 2011-2012 season, including criteria for patient
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign guidelines for submitting specimens

Edit your guidelines for submitting specimens form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your guidelines for submitting specimens form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit guidelines for submitting specimens online
Follow the guidelines below to benefit from the PDF editor's expertise:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit guidelines for submitting specimens. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
It's easier to work with documents with pdfFiller than you could have believed. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out guidelines for submitting specimens

How to fill out Guidelines for Submitting Specimens for Influenza 2011-2012
01
Begin by downloading the Guidelines for Submitting Specimens for Influenza 2011-2012 from the official website.
02
Review the document thoroughly to understand the submission criteria and requirements.
03
Identify the type of specimen you are submitting (e.g., nasal swab, throat swab, etc.).
04
Ensure that the specimen is collected using proper techniques to avoid contamination.
05
Label the specimen clearly with patient information, collection date, and type of specimen.
06
Complete any required forms that accompany the specimen submission, ensuring accuracy in all fields.
07
Package the specimen securely to prevent breakage or leakage during transport.
08
Send the specimen to the designated laboratory as specified in the guidelines.
Who needs Guidelines for Submitting Specimens for Influenza 2011-2012?
01
Healthcare providers who are involved in the collection and submission of influenza specimens.
02
Laboratories that perform testing for influenza and need guidelines for specimen submission.
03
Public health officials who are monitoring influenza trends and require proper specimen collection protocols.
Fill
form
: Try Risk Free






People Also Ask about
What is the protocol for influenza virus?
If you get sick with flu symptoms, in most cases, you should stay home and avoid contact with other people except to get medical care. If, however, you have symptoms of flu and are at increased risk for complications, are very sick with flu or worried about your illness, contact your health care provider right away.
What are the CDC guidelines for influenza?
Vaccination is the most effective way to prevent influenza and its complications. In the United States, CDC recommends annual seasonal influenza vaccination for people aged ≥6 months who do not have contraindications.
Do wound swabs need to be refrigerated?
Label swabs with patient's full name, date of birth, collection site(s), time and date of collection. Indicate self-collection if applicable. Ensure the lid is firmly placed/ on all collection devices containing specimens. Do not refrigerate swab specimens.
Do swabs need to be refrigerated?
Swabs for routine bacterial culture Place the swab in the tube containing Amies charcoal transport medium, and send it to the laboratory as soon as possible. If same day transport is not available then they can be stored in a refrigerator overnight but do not freeze.
Does the influenza vaccine need to be refrigerated?
Store influenza vaccine in the refrigerator at 35° to 46°F (2 to 8°C). Never expose influenza vaccine to freezing temperatures.
Do flu swabs need to be refrigerated?
Storing: Specimens should be placed into sterile viral transport media and immediately placed on refrigerant gel packs or at 4 degrees Celsius (refrigerator) for transport to the state public health laboratory. Keep specimens refrigerated (2-8 degrees Celsius, 26-46 degrees Fahrenheit) prior to shipping.
Do influenza swabs need to be refrigerated?
STORAGE: After collection, place the vial containing the swab and media into a zippered or sealable biohazard labeled bag. Keep the specimen(s) refrigerated at 4°C (39.2°F) until shipping.
What is the specimen for the flu virus?
A nasopharyngeal (NP) swab is the optimal upper respiratory tract specimen collection method for influenza testing. However, such specimens cannot be collected from infants and many older patients may not allow an NP specimen to be collected.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Guidelines for Submitting Specimens for Influenza 2011-2012?
The Guidelines for Submitting Specimens for Influenza 2011-2012 are comprehensive instructions that outline the procedures for collecting, handling, and sending specimens suspected of containing the influenza virus to laboratories for testing. These guidelines ensure that the specimens are suitable for accurate diagnosis and surveillance of the influenza virus.
Who is required to file Guidelines for Submitting Specimens for Influenza 2011-2012?
Healthcare providers, laboratories, and public health officials involved in the collection and submission of influenza specimens are required to adhere to the Guidelines for Submitting Specimens for Influenza 2011-2012.
How to fill out Guidelines for Submitting Specimens for Influenza 2011-2012?
To fill out the Guidelines for Submitting Specimens for Influenza 2011-2012, individuals should accurately complete all required fields, including patient information, specimen details, and relevant clinical history. Ensure clarity and legibility in writing, and follow any specific instructions included in the form regarding submission processes.
What is the purpose of Guidelines for Submitting Specimens for Influenza 2011-2012?
The purpose of the Guidelines for Submitting Specimens for Influenza 2011-2012 is to standardize the process of specimen collection and submission, thereby enhancing the accuracy and efficiency of influenza testing. This helps in effective disease surveillance and informs public health responses.
What information must be reported on Guidelines for Submitting Specimens for Influenza 2011-2012?
The information that must be reported includes the patient's demographics, clinical symptoms, date of specimen collection, type of specimen, laboratory handling information, and any relevant travel or exposure history related to influenza.
Fill out your guidelines for submitting specimens online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Guidelines For Submitting Specimens is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.