Get the free Listeriosis Investigation Guideline - kdheks
Show details
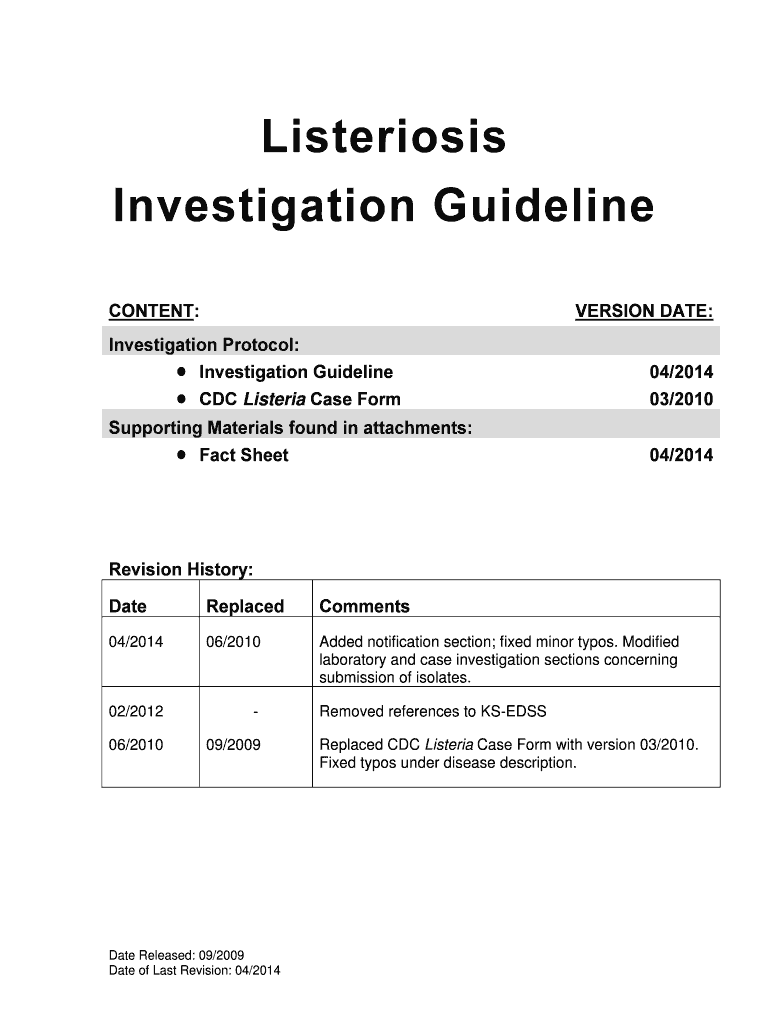
A guideline for the investigation and management of listeriosis cases, including definitions, laboratory criteria, treatment, epidemiology, and investigative responsibilities.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign listeriosis investigation guideline

Edit your listeriosis investigation guideline form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your listeriosis investigation guideline form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit listeriosis investigation guideline online
To use our professional PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit listeriosis investigation guideline. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
It's easier to work with documents with pdfFiller than you can have believed. Sign up for a free account to view.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out listeriosis investigation guideline

How to fill out Listeriosis Investigation Guideline
01
Begin by gathering necessary patient information such as demographics, medical history, and symptoms.
02
Document any recent food consumption history, focusing on high-risk foods.
03
Collect laboratory test results, including blood cultures that may indicate Listeria infection.
04
Fill out the sections related to epidemiological data, including contact with pregnant women, older adults, or immunocompromised individuals.
05
Include any outbreak information if applicable, noting the source and any linked cases.
06
Finalize the investigation by reviewing all sections for accuracy and completeness before submitting the document.
Who needs Listeriosis Investigation Guideline?
01
Healthcare professionals investigating potential cases of listeriosis.
02
Public health officials monitoring outbreaks of listeriosis.
03
Food safety inspectors assessing foodborne illness cases.
04
Laboratory personnel involved in the diagnosis and reporting of Listeria infections.
Fill
form
: Try Risk Free






People Also Ask about
What is the gold standard test for Listeria?
The current gold standard method for detection of infection with L. monocytogenes is through routine blood culture and Gram stain. Standard aerobic CSF cultures should be performed if CNS infection is suspected. This testing should be performed locally.
What testing method is used to diagnose listeriosis?
Clinical Testing Methods Testing for listeriosis requires routine bacterial culture of sterile site specimens (e.g. blood culture, cerebrospinal fluid).
What are the traditional methods of analysis for Listeria monocytogenes?
TL;DR: Conventional methods for the detection of Listeria monocytogenes in foods and environmental samples rely on selective pre-enrichment, enrichment, and plating, and confirmation of suspected colonies by testing a limited number of biochemical markers.
How do you diagnose listeriosis?
To diagnose listeriosis, doctors withdraw a sample of blood or do a spinal tap (lumbar puncture) to obtain a sample of the fluid that surrounds the brain and spinal cord (cerebrospinal fluid). The samples are sent to a laboratory to grow (culture) the bacteria.
What are the diagnostic tests for listeriosis?
Often, healthcare professionals do a blood test to find out if you have a listeria infection. Samples of spinal fluid might need to be tested as well. If you're pregnant, the fluid that surrounds your baby during pregnancy also might be tested.
What is the investigation for Listeria?
Listeria infection is confirmed when L. monocytogenes is identified, mostly by culture, from sterile sites (often cerebrospinal fluid or blood), foetus/neonate (including gastrointestinal contents) or associated products of conception (e.g. amniotic fluid, placental tissue).
What is the gold standard test for Listeria?
The current gold standard method for detection of infection with L. monocytogenes is through routine blood culture and Gram stain. Standard aerobic CSF cultures should be performed if CNS infection is suspected. This testing should be performed locally.
How to confirm Listeria monocytogenes?
The diagnosis of L. monocytogenes requires a culture of the bacteria from the blood, cerebral spinal fluid, or placental fluid. Once in the lab, Listeria species grows on a special type of agar called Meuller-Hinton agar. Culture will reveal gram-positive rods with colonies that are beta-hemolytic.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Listeriosis Investigation Guideline?
The Listeriosis Investigation Guideline is a set of procedures and protocols designed to investigate and manage cases of listeriosis, a serious infection caused by the bacterium Listeria monocytogenes.
Who is required to file Listeriosis Investigation Guideline?
Healthcare providers, laboratories, and public health officials are typically required to file the Listeriosis Investigation Guideline when a case of listeriosis is identified.
How to fill out Listeriosis Investigation Guideline?
To fill out the Listeriosis Investigation Guideline, provide detailed information about the patient, such as symptoms, laboratory results, potential sources of infection, and any exposure history.
What is the purpose of Listeriosis Investigation Guideline?
The purpose of the Listeriosis Investigation Guideline is to ensure timely identification and management of listeriosis cases to prevent outbreaks and protect public health.
What information must be reported on Listeriosis Investigation Guideline?
The information required includes patient demographics, clinical findings, laboratory results, possible sources of infection, and any relevant epidemiological links.
Fill out your listeriosis investigation guideline online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Listeriosis Investigation Guideline is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















