Get the free Application to Use Human Subjects in Research
Show details
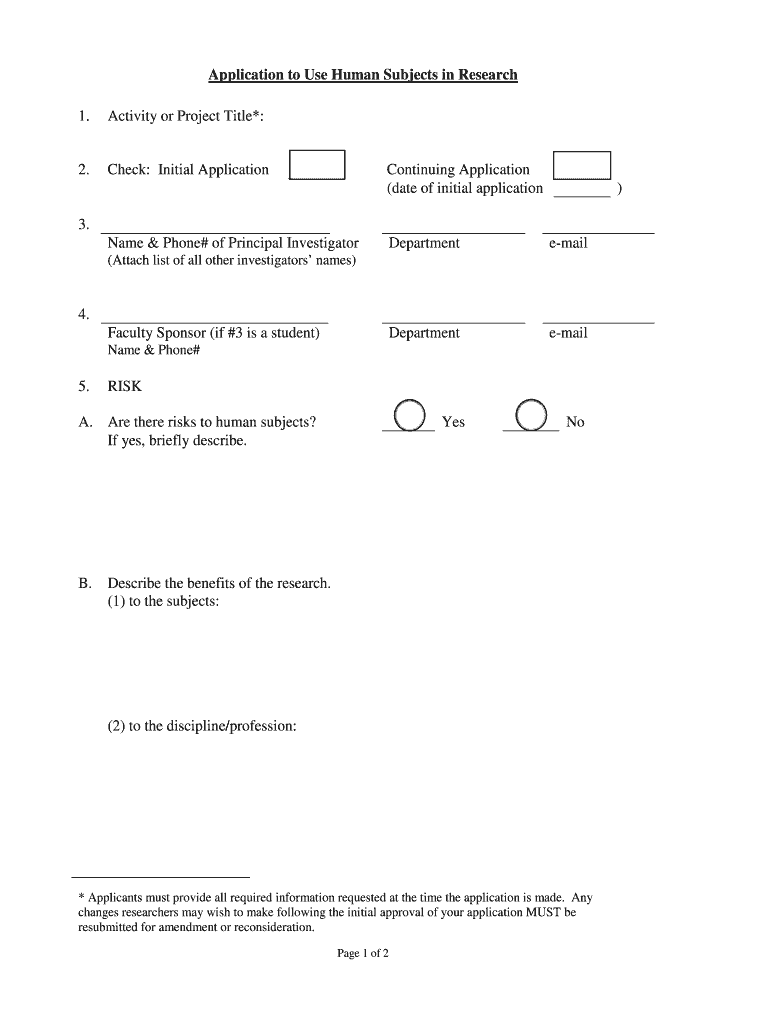
This document serves as an application form for researchers intending to use human subjects in their research, providing sections for project details, risk assessment, and informed consent requirements.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign application to use human

Edit your application to use human form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your application to use human form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing application to use human online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit application to use human. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Create an account to find out for yourself how it works!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out application to use human

How to fill out Application to Use Human Subjects in Research
01
Begin by gathering all necessary information about your research project.
02
Complete the application form with detailed information regarding your study's objectives, methodology, and participant recruitment.
03
Ensure that you outline the potential risks and benefits associated with the research.
04
Describe how you will obtain informed consent from participants.
05
Include details about the confidentiality measures you will implement to protect participant information.
06
Review your application for accuracy and completeness.
07
Submit the application to the appropriate review board for approval.
Who needs Application to Use Human Subjects in Research?
01
Researchers conducting studies involving human participants.
02
Academic institutions requiring ethical compliance for research projects.
03
Any organization that seeks to ensure the protection of human subjects in research.
Fill
form
: Try Risk Free






People Also Ask about
What are the rules for using humans in research studies?
Full unambiguous and informed consent from test subjects is required, except in extreme extenuating circumstances. Risks should be balanced out by potential benefits. Caution should be taken for subjects under 18 years old. Extreme caution should be taken if microorganisms are involved.
What are the guidelines for doing research with humans?
Respect for potential and enrolled participants This includes: respecting their privacy and keeping their private information confidential. respecting their right to change their mind, to decide that the research does not match their interests, and to withdraw without a penalty.
How can we protect human subjects in research?
Respect for persons Informed consent. Protecting privacy and maintaining confidentiality. Additional safeguards for protection of subjects likely to be vulnerable to coercion (e.g. threats of harm) or undue influence (e.g. excessive compensation)
When using human subjects, what must researchers ensure?
As such, all research involving human beings should be reviewed by an ethics committee to ensure that the appropriate ethical standards are being upheld. Discussion of the ethical principles of beneficence, justice and autonomy are central to ethical review.
Which of the following is a consideration when conducting research using human subjects?
Applications of the general principles to the conduct of research leads to consideration of the following requirements: informed consent, risk/benefit assessment, and the selection of subjects of research.
What steps must a researcher take when dealing with human subjects?
Respect for persons Informed consent. Protecting privacy and maintaining confidentiality. Additional safeguards for protection of subjects likely to be vulnerable to coercion (e.g. threats of harm) or undue influence (e.g. excessive compensation)
What is a key requirement of human subjects research?
You will need to get IRB or IEC approval of your human subjects research, including the protocol, informed consent document, and possibly other documents. Where other institutions are involved in the research, e.g., a multicenter study, you must comply with the NIH Single IRB Policy for Multi-Site Research.
What is the Common Rule for research involving human subjects?
The Common Rule includes additional protections for certain vulnerable research subjects: Subpart B provides additional protections for pregnant women, in vitro fertilization, and fetuses. Subpart C contains additional protections for prisoners. Subpart D does the same for children.
What are the criteria for human subjects research?
It must include human subjects, which are living people about whom an investigator conducting research either a) obtains information or biospecimens through intervention or interaction with the individual and uses, studies, or analyzes the information or biospecimens; or b) obtains, uses, studies, analyzes, or
What are the advantages of using human subjects in research?
The use of human subjects in research benefits society in many ways, from contributing to the development of new drugs and medical procedures to understanding how we think and act. It also can and has imposed unacceptable risks on research subjects.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Application to Use Human Subjects in Research?
An Application to Use Human Subjects in Research is a formal document that researchers submit to seek approval for conducting studies that involve human participants, ensuring ethical standards and participant safety.
Who is required to file Application to Use Human Subjects in Research?
Researchers, faculty, or students who plan to conduct studies that involve human participants are required to file an Application to Use Human Subjects in Research.
How to fill out Application to Use Human Subjects in Research?
To fill out the Application to Use Human Subjects in Research, researchers typically need to provide detailed information about the study's purpose, methodology, participant recruitment, informed consent process, and data handling procedures.
What is the purpose of Application to Use Human Subjects in Research?
The purpose of this application is to ensure that research involving human subjects is conducted ethically, protecting the rights and welfare of participants while ensuring compliance with legal and institutional guidelines.
What information must be reported on Application to Use Human Subjects in Research?
Essential information that must be reported includes the study title, research objectives, participant demographics, potential risks and benefits, informed consent procedures, and data protection measures.
Fill out your application to use human online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Application To Use Human is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















