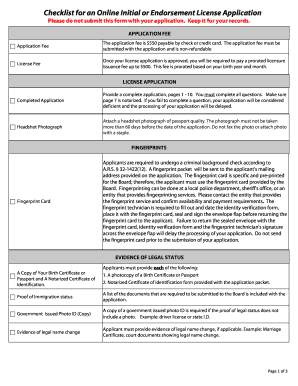
Get the free A Phase II Study of Total Skin Electron Beam Therapy (TSEBT) to dose of 12 Gy in sta...
Show details
This document outlines a clinical trial protocol evaluating the efficacy and safety of Total Skin Electron Beam Therapy (TSEBT) at a total dose of 12 Gy for patients with stage IB-IIIA mycosis fungoides.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign a phase ii study

Edit your a phase ii study form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your a phase ii study form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing a phase ii study online
Follow the guidelines below to benefit from a competent PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit a phase ii study. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
Dealing with documents is simple using pdfFiller.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out a phase ii study

How to fill out A Phase II Study of Total Skin Electron Beam Therapy (TSEBT) to dose of 12 Gy in stage IB-IIIA mycosis fungoides
01
Obtain informed consent from the patient after explaining the study details.
02
Confirm the patient's diagnosis of stage IB-IIIA mycosis fungoides.
03
Conduct a thorough medical evaluation to determine eligibility.
04
Randomly assign the patient to the treatment group for TSEBT at a dose of 12 Gy.
05
Schedule the patient for imaging and baseline assessments.
06
Begin treatment with the total skin electron beam therapy, ensuring the dosage is accurately delivered.
07
Monitor the patient regularly for treatment effects and side effects.
08
Document all patient responses and any adverse reactions during the study.
09
Follow-up with the patient post-treatment for additional assessments and to collect long-term data.
Who needs A Phase II Study of Total Skin Electron Beam Therapy (TSEBT) to dose of 12 Gy in stage IB-IIIA mycosis fungoides?
01
Patients diagnosed with stage IB-IIIA mycosis fungoides who require treatment options.
02
Individuals seeking clinical trial participation for innovative therapies in skin lymphomas.
03
Doctors and clinicians looking for evidence-based options for managing mycosis fungoides.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is A Phase II Study of Total Skin Electron Beam Therapy (TSEBT) to dose of 12 Gy in stage IB-IIIA mycosis fungoides?
A Phase II Study of Total Skin Electron Beam Therapy (TSEBT) to dose of 12 Gy in stage IB-IIIA mycosis fungoides is a clinical research trial that investigates the effectiveness and safety of delivering a total dose of 12 Gy of electron beam radiation to the skin in patients diagnosed with stage IB-IIIA mycosis fungoides, which is a form of cutaneous T-cell lymphoma.
Who is required to file A Phase II Study of Total Skin Electron Beam Therapy (TSEBT) to dose of 12 Gy in stage IB-IIIA mycosis fungoides?
Principal investigators, usually oncologists or researchers leading the study, are required to file the study protocol and related documents with regulatory authorities, including ethical review boards and clinical trial registries.
How to fill out A Phase II Study of Total Skin Electron Beam Therapy (TSEBT) to dose of 12 Gy in stage IB-IIIA mycosis fungoides?
To fill out the study documentation, researchers should provide detailed information about the study design, patient eligibility criteria, treatment protocols, assessment methods, data collection strategies, and analysis plans, ensuring compliance with ethical and regulatory guidelines.
What is the purpose of A Phase II Study of Total Skin Electron Beam Therapy (TSEBT) to dose of 12 Gy in stage IB-IIIA mycosis fungoides?
The purpose of the study is to assess the efficacy, safety, and tolerability of a 12 Gy dose of TSEBT in treating patients with stage IB-IIIA mycosis fungoides, aiming to improve patient outcomes and inform future treatment strategies.
What information must be reported on A Phase II Study of Total Skin Electron Beam Therapy (TSEBT) to dose of 12 Gy in stage IB-IIIA mycosis fungoides?
Information that must be reported includes patient demographics, treatment dosages and schedules, adverse events, treatment outcomes, response rates, and any other relevant clinical data that can provide insight into the effectiveness and safety of the therapy.
Fill out your a phase ii study online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

A Phase Ii Study is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.