Get the free University of Pittsburgh Research Conduct and Compliance Office Audit Report
Show details
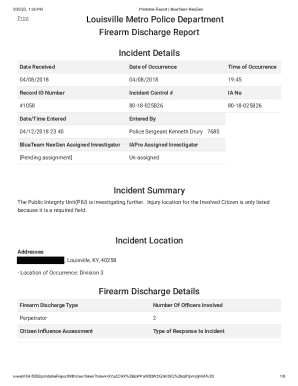
This document outlines the procedures and findings of an audit conducted by the University of Pittsburgh Research Conduct and Compliance Office regarding a specific research protocol. It covers various
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign university of pittsburgh research

Edit your university of pittsburgh research form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your university of pittsburgh research form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing university of pittsburgh research online
Follow the guidelines below to benefit from the PDF editor's expertise:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit university of pittsburgh research. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
It's easier to work with documents with pdfFiller than you could have ever thought. You may try it out for yourself by signing up for an account.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out university of pittsburgh research

How to fill out University of Pittsburgh Research Conduct and Compliance Office Audit Report
01
Step 1: Gather all necessary documentation related to your research project.
02
Step 2: Review the guidelines provided by the University of Pittsburgh Research Conduct and Compliance Office.
03
Step 3: Complete the personal information section, including your name, department, and project details.
04
Step 4: Outline the research methods and compliance measures you have implemented.
05
Step 5: List any previous audits or compliance checks that have been conducted.
06
Step 6: Provide a detailed account of any issues or incidents that occurred during the research.
07
Step 7: Submit the completed report by the specified deadline to the appropriate office.
Who needs University of Pittsburgh Research Conduct and Compliance Office Audit Report?
01
Researchers conducting studies at the University of Pittsburgh.
02
Administrative staff responsible for compliance and oversight of research activities.
03
Institutional Review Board (IRB) members assessing research protocols.
Fill
form
: Try Risk Free






People Also Ask about
What are the key principles for IRB approval?
When reviewing research, Institutional Review Board (IRB) members are guided by three ethical principles that are fundamental to human participant protection: respect for persons, beneficence, and justice.
What information must be included in the research proposal that is submitted to the IRB?
All proposals submitted for either expedited or full review must contain four primary sections: Purpose of investigation and procedures. Anticipated risk and potential benefits to participants. Steps taken to protect the participants.
What are the steps of the IRB process?
IRB Process Submission of Research Proposal: Researchers submit their research proposal to the IRB. Initial Review: Exemption Determination (if applicable): Expedited Review (if applicable): Full Board Review (if applicable): Approval Decision: Ongoing Oversight:
What are the 3 levels of investigation review from the IRB when submitting information for a research study?
IRB must review all projects that meet the definition of research and that involve human subjects prior to any data collection to determine the appropriate level of review, and, as appropriate, approve them. There are three major types of review: Exempt, Expedited, and Full.
What is the Pitt Concern Connection?
The Pitt Concern Connection enables our campus communities to elevate irregular or troublesome workplace, academic, and other issues so that they can be reviewed, addressed, and resolved. Report an issue or ask a question online, by telephone or via text message.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is University of Pittsburgh Research Conduct and Compliance Office Audit Report?
The University of Pittsburgh Research Conduct and Compliance Office Audit Report is a document that assesses compliance with federal, state, and institutional regulations regarding research practices. It is designed to ensure that research activities are conducted ethically and in accordance with established guidelines.
Who is required to file University of Pittsburgh Research Conduct and Compliance Office Audit Report?
All faculty, staff, and researchers involved in funded research projects at the University of Pittsburgh are typically required to file the University of Pittsburgh Research Conduct and Compliance Office Audit Report to demonstrate adherence to guidelines and regulations.
How to fill out University of Pittsburgh Research Conduct and Compliance Office Audit Report?
To fill out the University of Pittsburgh Research Conduct and Compliance Office Audit Report, individuals must gather relevant data about their research activities, including funding sources, project descriptions, compliance with ethical considerations, and any reported incidents. The report should be completed following the prescribed format and submitted by the specified deadline.
What is the purpose of University of Pittsburgh Research Conduct and Compliance Office Audit Report?
The purpose of the University of Pittsburgh Research Conduct and Compliance Office Audit Report is to ensure that research activities comply with ethical standards and regulatory requirements, promote accountability among researchers, and enhance the overall integrity of research conducted at the institution.
What information must be reported on University of Pittsburgh Research Conduct and Compliance Office Audit Report?
The information that must be reported on the University of Pittsburgh Research Conduct and Compliance Office Audit Report typically includes research project title, principal investigator details, funding sources, compliance with federal and institutional policies, any issues or violations encountered, and status updates on corrective actions taken.
Fill out your university of pittsburgh research online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

University Of Pittsburgh Research is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.