Get the free Unit 5 Day 6 Activity – Solubility - ctp cm utexas
Show details
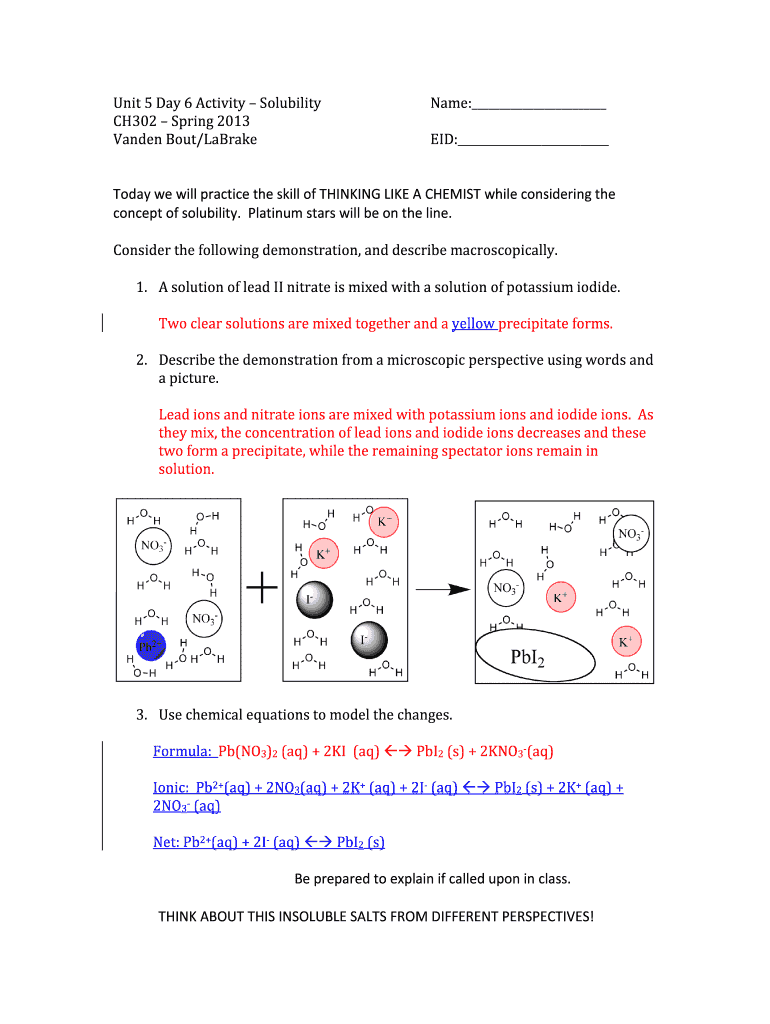
This document contains an educational activity focused on the solubility concept in chemistry, involving demonstrations, calculations, and explanations regarding lead II nitrate and potassium iodide
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign unit 5 day 6

Edit your unit 5 day 6 form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your unit 5 day 6 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing unit 5 day 6 online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit unit 5 day 6. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out unit 5 day 6

How to fill out Unit 5 Day 6 Activity – Solubility
01
Gather all necessary materials: Unit 5 Day 6 Activity worksheet, pencil, and any reference materials.
02
Read the instructions at the top of the worksheet carefully.
03
Begin with the first section, which may ask for definitions or examples related to solubility.
04
Use your textbook or notes to provide accurate definitions or examples.
05
Move on to the next section that may include solving problems or completing charts related to solubility.
06
Ensure that you show all calculations for problem-solving sections and check your work.
07
Complete any questions that require reflection or explanation of concepts.
08
Review your answers for accuracy and completeness before submitting.
Who needs Unit 5 Day 6 Activity – Solubility?
01
Students learning about chemistry concepts, particularly solubility.
02
Teachers looking for a structured activity to assess student understanding of solubility.
03
Parents supporting their children’s learning in science.
Fill
form
: Try Risk Free






People Also Ask about
What is solubility for class 6?
What is Solubility? The maximum amount of solute that can dissolve in a known quantity of solvent at a certain temperature is its solubility.
How does solubility get affected on increasing the temperature?
Increasing solubility with increasing temperature Increasing the temperature, therefore, increases the solubility of the solute. An example of a solute whose solubility increases with greater temperature is ammonium nitrate, which can be used in first-aid cold packs.
What is soluble for class 6?
the material which gets dissolved in water are called soluble substance. the material which donot gets dissolved in water is called insoluble substance.
What is solubility class 6 pdf?
Solubility is. kind of the property of a liquid, solid, and gaseous chemical. substance called solute to dissolve in a liquid, solid, and. gaseous solvent to form a homogeneous solution of the. solute in the solvent.
What is solubility pdf?
Solubility is. kind of the property of a liquid, solid, and gaseous chemical. substance called solute to dissolve in a liquid, solid, and. gaseous solvent to form a homogeneous solution of the. solute in the solvent.
What is solubility 6?
Solubility refers to the ability of a substance to dissolve in another substance, usually a liquid. In scientific terms, it often refers to the maximum amount of solute that can be dissolved in a given amount of solvent at a specific temperature.
What is solubility?
solubility, degree to which a substance dissolves in a solvent to make a solution (usually expressed as grams of solute per litre of solvent). Solubility of one fluid (liquid or gas) in another may be complete (totally miscible; e.g., methanol and water) or partial (oil and water dissolve only slightly).
What is solubility ks3?
Solubility is the term used to describe how easy it is for a substance to dissolve into a liquid (solvent). If a substance dissolves easily, like salt into water, then it is highly soluble. Some materials are insoluble, like flour and sand, meaning they do not dissolve in water.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Unit 5 Day 6 Activity – Solubility?
Unit 5 Day 6 Activity – Solubility is an educational activity designed to help students understand the concept of solubility, including how substances dissolve in solvents, the factors that affect solubility, and practical applications in real-world scenarios.
Who is required to file Unit 5 Day 6 Activity – Solubility?
Students participating in the Unit 5 curriculum are typically required to complete and file the Unit 5 Day 6 Activity – Solubility as part of their coursework to demonstrate their understanding of the related concepts.
How to fill out Unit 5 Day 6 Activity – Solubility?
To fill out Unit 5 Day 6 Activity – Solubility, students should follow the provided instructions, which usually include responding to prompts regarding their observations, conclusions about solubility, and any data collected during experiments.
What is the purpose of Unit 5 Day 6 Activity – Solubility?
The purpose of Unit 5 Day 6 Activity – Solubility is to engage students in exploring and understanding the principles of solubility, encouraging hands-on experimentation and critical thinking about the interactions between different substances.
What information must be reported on Unit 5 Day 6 Activity – Solubility?
Students must report their experimental observations, the results of any solubility tests conducted, relevant data such as temperature and concentration, and their conclusions regarding the factors influencing solubility.
Fill out your unit 5 day 6 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Unit 5 Day 6 is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















