FDA 3537 2008 free printable template
Show details
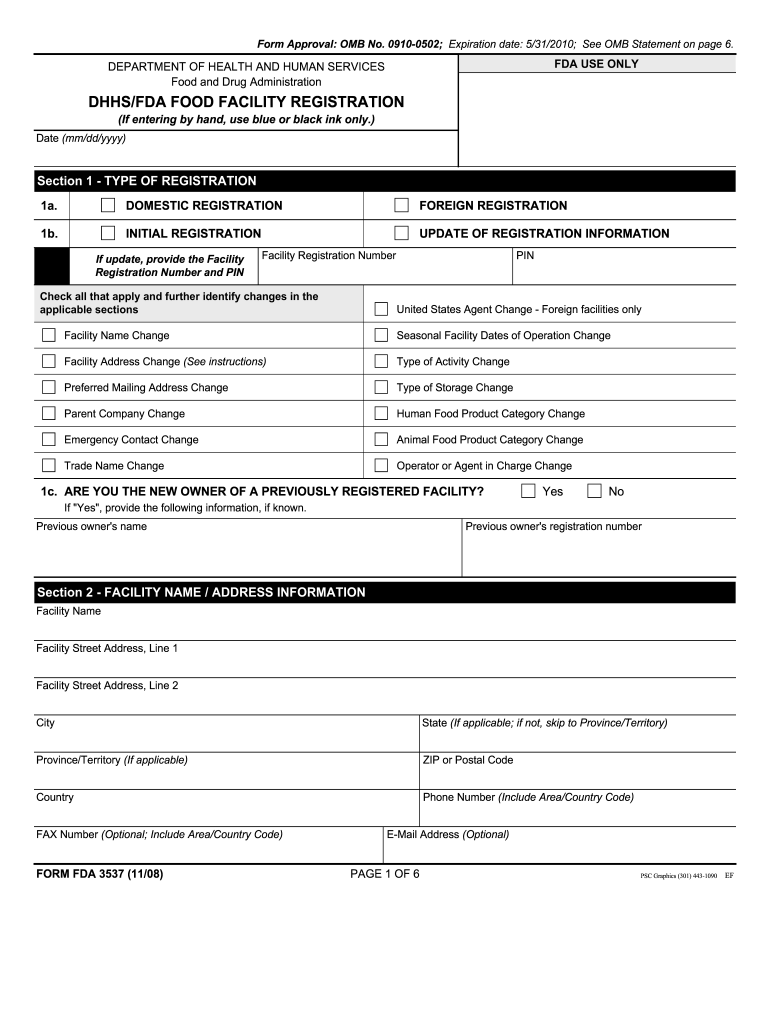
Form Approval: OMB No. 0910-0502; Expiration date: 5/31/2010; See OMB Statement on page 6. DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration FDA USE ONLY HHS/FDA FOOD FACILITY REGISTRATION
pdfFiller is not affiliated with any government organization
Get, Create, Make and Sign FDA 3537

Edit your FDA 3537 form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your FDA 3537 form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing FDA 3537 online
Use the instructions below to start using our professional PDF editor:
1
Log in to account. Start Free Trial and register a profile if you don't have one yet.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit FDA 3537. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
FDA 3537 Form Versions
Version
Form Popularity
Fillable & printabley
How to fill out FDA 3537

How to fill out FDA 3537
01
Download the FDA 3537 form from the FDA website.
02
Fill in your personal information at the top section, including your name, address, and contact information.
03
Provide details about the product, including the product name, type, and intended use.
04
Indicate the regulatory status of the product and any relevant identifiers, such as the NDC (National Drug Code) if applicable.
05
Complete the sections regarding the manufacturer or applicant, including name and address.
06
If applicable, include any additional information related to the product’s safety or efficacy.
07
Review all entries for accuracy and completeness.
08
Sign and date the form at the end.
09
Submit the completed form according to the specified submission guidelines on the FDA website.
Who needs FDA 3537?
01
Manufacturers of drugs, biologics, or medical devices in the United States.
02
Applicants seeking FDA approval for new products.
03
Companies involved in the marketing or distribution of products that require specific regulatory oversight.
Fill
form
: Try Risk Free






People Also Ask about
Do you have to pay to get FDA approval?
An application fee is due when the application is submitted to FDA. FDA issues invoices for annual program fees for the coming fiscal year in August of each year using the fee schedule for the coming fiscal year.
What is FDA food facility registration?
Food facility registration will help FDA to: • Determine the location and source of a potential bioterrorism incident or an outbreak of food-borne illness; and • Quickly notify facilities that may be affected. There is no fee for registration or updates to a registration.
How much does an FDA certificate cost?
U.S. FDA Food Facility Registration for Food Beverage and Dietary Supplements fee 2022. Service & Fees: Food Facility Registration, 395 USD. U.S. Agent Representation, Free.
How do I renew my FDA food facility registration?
Log into the FDA Industry Systems (FIS). Choose "FURLS Food Facility Registration Module (FFRM)" from the list of available systems on Account Management Home Page. Choose the "Update Facility Registration” option from the FFR main menu.
How do I get my product FDA certified?
To get FDA approval, drug manufacturers must conduct lab, animal, and human clinical testing and submit their data to FDA. FDA will then review the data and may approve the drug if the agency determines that the benefits of the drug outweigh the risks for the intended use.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I edit FDA 3537 online?
The editing procedure is simple with pdfFiller. Open your FDA 3537 in the editor, which is quite user-friendly. You may use it to blackout, redact, write, and erase text, add photos, draw arrows and lines, set sticky notes and text boxes, and much more.
Can I create an eSignature for the FDA 3537 in Gmail?
With pdfFiller's add-on, you may upload, type, or draw a signature in Gmail. You can eSign your FDA 3537 and other papers directly in your mailbox with pdfFiller. To preserve signed papers and your personal signatures, create an account.
Can I edit FDA 3537 on an Android device?
You can. With the pdfFiller Android app, you can edit, sign, and distribute FDA 3537 from anywhere with an internet connection. Take use of the app's mobile capabilities.
What is FDA 3537?
FDA 3537 is a form used to report the manufacturing and distribution of medical devices under the FDA's regulations.
Who is required to file FDA 3537?
Manufacturers and importers of medical devices are required to file FDA 3537.
How to fill out FDA 3537?
FDA 3537 must be filled out by providing detailed information regarding the device, such as device classification, intended use, and any adverse events associated with the device.
What is the purpose of FDA 3537?
The purpose of FDA 3537 is to ensure the FDA has the necessary information to monitor the safety and effectiveness of medical devices in the market.
What information must be reported on FDA 3537?
Information that must be reported includes device identification, manufacturer details, distribution data, and any incident reports related to the device.
Fill out your FDA 3537 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

FDA 3537 is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.























