Get the free Institutional Review Board - Greater Baltimore Medical Center - gbmc
Show details
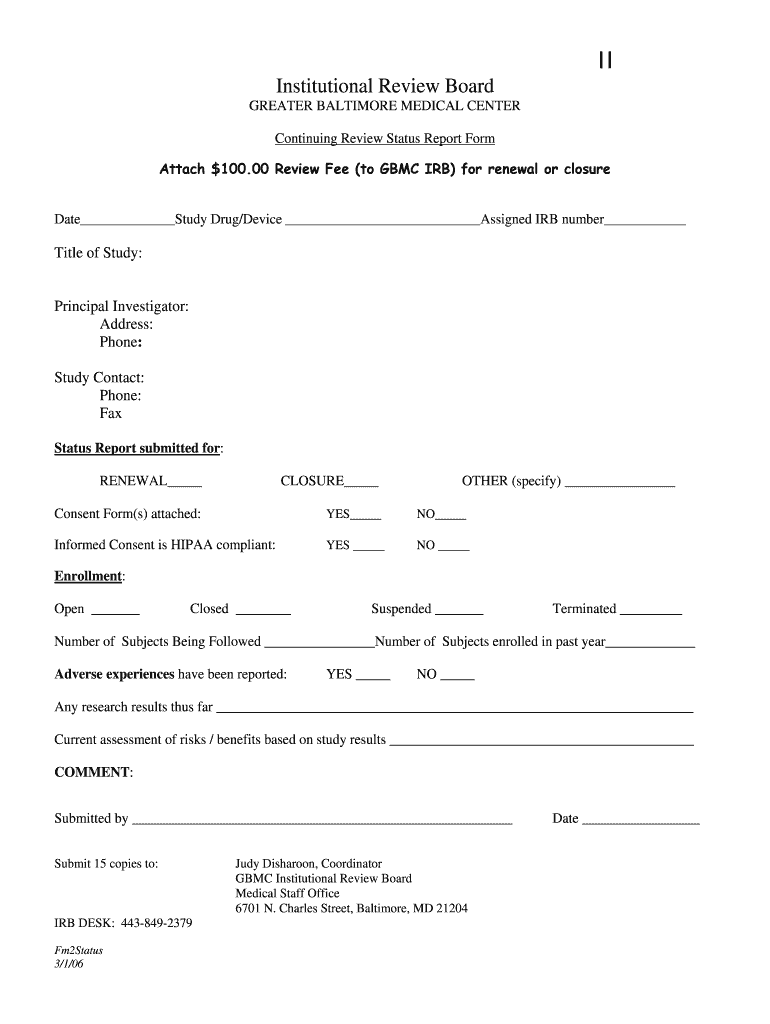
II Institutional Review Board GREATER BALTIMORE MEDICAL CENTER Continuing Review Status Report Form Attach $100.00 Review Fee (to GBM CIRB) for renewal or closure Date Study Drug/Device Assigned IRB
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign institutional review board

Edit your institutional review board form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your institutional review board form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit institutional review board online
Follow the steps down below to benefit from a competent PDF editor:
1
Log in to account. Start Free Trial and sign up a profile if you don't have one yet.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit institutional review board. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out institutional review board

How to fill out institutional review board?
01
Start by gathering all necessary documents and information required by the institutional review board (IRB). This may include your research proposal, consent forms, participant recruitment materials, and any supporting documents.
02
Carefully read and understand the IRB application form or online submission platform. Pay attention to specific instructions, guidelines, and requirements outlined by the IRB.
03
Complete all sections of the application form accurately, providing clear and concise information. Include details about the research aims, methods, data collection procedures, and potential risks or benefits to participants.
04
Ensure that all informed consent procedures are appropriately described and that consent forms meet the necessary ethical standards. Include information on confidentiality, participant rights, and how consent will be documented.
05
If applicable, specify any additional safeguards or measures you will put in place to protect vulnerable populations, such as children, prisoners, or individuals with limited decision-making capacity.
06
Attach any supporting documents, such as a detailed research protocol, interview guides, survey instruments, or recruitment materials. Make sure all attachments are labeled clearly and organized in a logical order.
07
Double-check that all required signatures have been obtained, including those of the principal investigator, faculty advisor (if applicable), and any co-investigators or collaborators involved in the project.
08
Review the completed application form and attachments for accuracy, consistency, and adherence to the IRB's guidelines and requirements. Make any necessary revisions or additions before submitting the application.
09
Submit the completed application and accompanying documents to the designated IRB office or online submission platform. Keep copies of all materials for your own records.
10
After submission, follow up with the IRB to ensure that your application has been received and is being reviewed. Be prepared to address any clarifications or modifications requested by the IRB.
Who needs institutional review board?
01
Researchers conducting studies involving human participants, regardless of the field or discipline, may need to undergo IRB review. This includes both academic and non-academic researchers.
02
Institutional review boards are typically required by universities, hospitals, and other organizations that receive federal funding or are committed to upholding ethical standards in research involving human subjects.
03
It is important to note that not all research projects require IRB review. However, if your study involves interactions or interventions with living individuals, collection of identifiable private information, or involves a vulnerable population, it is likely that IRB review will be necessary.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit institutional review board from Google Drive?
By integrating pdfFiller with Google Docs, you can streamline your document workflows and produce fillable forms that can be stored directly in Google Drive. Using the connection, you will be able to create, change, and eSign documents, including institutional review board, all without having to leave Google Drive. Add pdfFiller's features to Google Drive and you'll be able to handle your documents more effectively from any device with an internet connection.
Can I create an electronic signature for the institutional review board in Chrome?
Yes. By adding the solution to your Chrome browser, you may use pdfFiller to eSign documents while also enjoying all of the PDF editor's capabilities in one spot. Create a legally enforceable eSignature by sketching, typing, or uploading a photo of your handwritten signature using the extension. Whatever option you select, you'll be able to eSign your institutional review board in seconds.
Can I edit institutional review board on an Android device?
With the pdfFiller mobile app for Android, you may make modifications to PDF files such as institutional review board. Documents may be edited, signed, and sent directly from your mobile device. Install the app and you'll be able to manage your documents from anywhere.
What is institutional review board?
The institutional review board (IRB) is a committee responsible for reviewing and approving research protocols to ensure the protection of the rights and welfare of human research subjects.
Who is required to file institutional review board?
Researchers and institutions conducting human subjects research are required to file with the institutional review board.
How to fill out institutional review board?
To fill out the IRB, researchers must submit their research protocol, informed consent forms, and any other relevant documents for review by the IRB committee.
What is the purpose of institutional review board?
The purpose of the institutional review board is to ensure that research involving human subjects is conducted ethically and in compliance with regulations to protect the rights and welfare of the research participants.
What information must be reported on institutional review board?
Researchers must report details about the study design, risks and benefits to participants, informed consent process, recruitment methods, and plans for data management and confidentiality.
Fill out your institutional review board online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Institutional Review Board is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















