Get the free Center for Clinical Investigation Booking Information - brighamandwomens
Show details
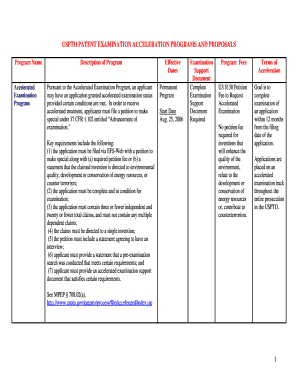
Center for Clinical Investigation Booking Information Protocol Number: Protocol Title: Booked By: Telephone: Patient Name: Medical Record #: Last Name, Address: First Name Telephone: Street Date of
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign center for clinical investigation

Edit your center for clinical investigation form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your center for clinical investigation form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing center for clinical investigation online
Follow the guidelines below to take advantage of the professional PDF editor:
1
Check your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit center for clinical investigation. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, it's always easy to work with documents. Check it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out center for clinical investigation

How to fill out a center for clinical investigation?
01
Start by gathering the necessary information and documents, such as the study protocol, informed consent forms, and any other relevant study materials.
02
Determine the study objectives and research question that the center for clinical investigation aims to address. This will help guide the entire process.
03
Develop a comprehensive plan for participant recruitment. This may involve advertising the study through various channels, reaching out to potential participants, and screening them for eligibility.
04
Establish a screening process to assess the suitability of potential participants. This may include reviewing medical records, conducting physical examinations, and performing diagnostic tests.
05
Obtain informed consent from eligible participants. Make sure to explain the study procedures, potential risks and benefits, and any alternatives for participation. Ensure that participants have a clear understanding and provide their voluntary consent.
06
Assign participants to different study groups or treatment arms, if applicable. Randomization should be performed using appropriate methods to ensure unbiased allocation.
07
Implement the study protocol and perform the necessary procedures. This may include administration of investigational drugs, collection of biological samples, or conducting specific tests or assessments.
08
Monitor participants closely throughout the study period to ensure adherence to the protocol and capture accurate data. This may involve regular check-ups, scheduled visits, or remote monitoring techniques.
09
Collect and analyze the data collected during the study. Ensure that all data is accurately recorded, stored securely, and analyzed according to the study protocol and statistical analysis plan.
10
Summarize the study findings in a comprehensive report, highlighting the results, conclusions, and any potential implications. This report may be used for publication in scientific journals, presenting at conferences, or informing regulatory authorities.
Who needs a center for clinical investigation?
01
Pharmaceutical companies: Pharmaceutical companies often require a center for clinical investigation to conduct clinical trials for new drugs or therapies. These centers play a crucial role in ensuring the safety and efficacy of the investigational products.
02
Academic institutions: Universities and research institutions may establish centers for clinical investigation to facilitate medical research and contribute to scientific knowledge. These centers often collaborate with pharmaceutical companies or government agencies to conduct clinical studies.
03
Healthcare providers: Hospitals and medical centers may set up centers for clinical investigation to participate in research studies. This allows them to contribute to medical advancements, provide patients with access to innovative therapies, and enhance their reputation as research-intensive institutions.
04
Government agencies: Regulatory bodies and government research organizations may establish centers for clinical investigation to oversee and conduct studies necessary for the evaluation of new drugs, medical devices, or public health interventions.
05
Non-profit organizations: Non-profit organizations focused on specific diseases or conditions may establish centers for clinical investigation to support research efforts aimed at improving patient outcomes and advancing medical knowledge in their respective fields.
In summary, filling out a center for clinical investigation involves gathering the necessary documentation, developing a study plan, recruiting participants, managing the study procedures, collecting and analyzing data, and reporting the findings. The center is needed by various stakeholders such as pharmaceutical companies, academic institutions, healthcare providers, government agencies, and non-profit organizations.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit center for clinical investigation on a smartphone?
The pdfFiller apps for iOS and Android smartphones are available in the Apple Store and Google Play Store. You may also get the program at https://edit-pdf-ios-android.pdffiller.com/. Open the web app, sign in, and start editing center for clinical investigation.
How do I fill out the center for clinical investigation form on my smartphone?
Use the pdfFiller mobile app to fill out and sign center for clinical investigation on your phone or tablet. Visit our website to learn more about our mobile apps, how they work, and how to get started.
How do I edit center for clinical investigation on an Android device?
You can edit, sign, and distribute center for clinical investigation on your mobile device from anywhere using the pdfFiller mobile app for Android; all you need is an internet connection. Download the app and begin streamlining your document workflow from anywhere.
What is center for clinical investigation?
Center for clinical investigation is a facility where clinical research studies are conducted on human participants.
Who is required to file center for clinical investigation?
The principal investigator or the sponsor of the clinical research study is required to file the center for clinical investigation.
How to fill out center for clinical investigation?
Center for clinical investigation can be filled out online through the designated platform or submitted via mail with the required documentation.
What is the purpose of center for clinical investigation?
The purpose of center for clinical investigation is to ensure transparency and compliance with regulations in the conduct of clinical research studies.
What information must be reported on center for clinical investigation?
Center for clinical investigation must include information on the study protocol, informed consent process, facilities involved, and any potential risks to participants.
Fill out your center for clinical investigation online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Center For Clinical Investigation is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.