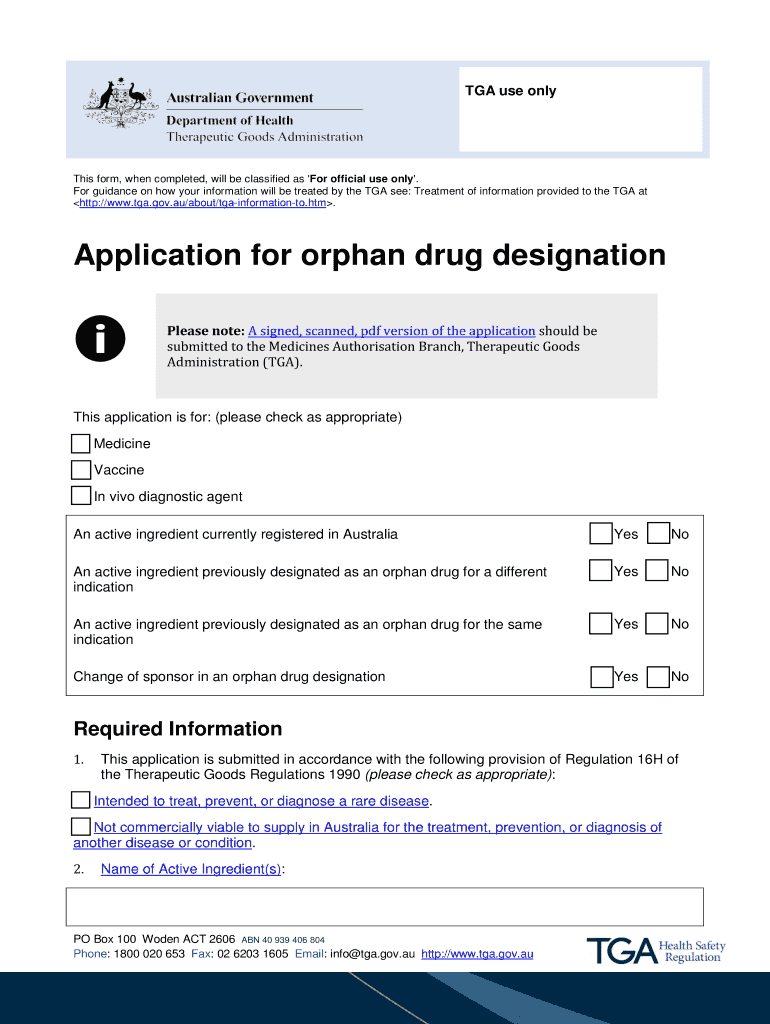
Get the free BApplicationb for orphan drug designation - Therapeutic Goods bb - tga gov
Show details
TGA use only This form, when completed, will be classified as 'For official use only '. For guidance on how your information will be treated by the TGA see: Treatment of information provided to the
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign bapplicationb for orphan drug

Edit your bapplicationb for orphan drug form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your bapplicationb for orphan drug form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit bapplicationb for orphan drug online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit bapplicationb for orphan drug. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out bapplicationb for orphan drug

How to fill out an application for orphan drug:
01
Gather all necessary documents and information: Before starting the application process, make sure you have all the required documents and information ready. This may include details about the drug, medical evidence, clinical trial data, and any other relevant documentation.
02
Research and familiarize yourself with the regulatory requirements: It is crucial to understand the specific regulatory requirements for orphan drug applications in your region or country. This information can be obtained from regulatory authorities or relevant organizations involved in orphan drug development.
03
Fill out the application form: Begin by carefully reading and understanding each section of the application form. Fill in all the required fields accurately and provide detailed information to support your case for orphan drug designation. It is important to provide evidence of the drug's potential to treat a rare disease or condition.
04
Include supportive documents: Attach all necessary supporting documents, such as preclinical and clinical trial data, safety and efficacy information, and any other relevant documentation that strengthens your application. These documents should clearly demonstrate the drug's potential benefits and its uniqueness in treating a rare disease or condition.
05
Seek professional advice, if needed: If you find the application process complex or challenging, consider seeking advice from professionals experienced in orphan drug development. They can provide guidance, review your application, and suggest any improvements needed to increase your chances of success.
Who needs an application for orphan drug?
01
Pharmaceutical companies: Companies involved in orphan drug development need to fill out an application to obtain orphan drug designation for their potential treatments. This designation provides various benefits, such as market exclusivity and financial incentives, which can incentivize the development of drugs for rare diseases.
02
Researchers and scientists: Individuals working on the development of potential orphan drugs also need to complete the application process. They are responsible for gathering the necessary data and documentation to support the drug's potential benefits and its relevance to treating a rare disease or condition.
03
Regulatory authorities: Government regulatory authorities are involved in reviewing and approving orphan drug applications. They play a crucial role in ensuring that the proposed drug meets the specific criteria and requirements for orphan drug designation.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit bapplicationb for orphan drug from Google Drive?
You can quickly improve your document management and form preparation by integrating pdfFiller with Google Docs so that you can create, edit and sign documents directly from your Google Drive. The add-on enables you to transform your bapplicationb for orphan drug into a dynamic fillable form that you can manage and eSign from any internet-connected device.
Where do I find bapplicationb for orphan drug?
The premium pdfFiller subscription gives you access to over 25M fillable templates that you can download, fill out, print, and sign. The library has state-specific bapplicationb for orphan drug and other forms. Find the template you need and change it using powerful tools.
How do I fill out bapplicationb for orphan drug on an Android device?
Use the pdfFiller Android app to finish your bapplicationb for orphan drug and other documents on your Android phone. The app has all the features you need to manage your documents, like editing content, eSigning, annotating, sharing files, and more. At any time, as long as there is an internet connection.
What is application for orphan drug?
An application for orphan drug is a request submitted to the regulatory authority for the designation, approval, or marketing of a drug intended to treat a rare medical condition.
Who is required to file application for orphan drug?
Any pharmaceutical company or sponsor developing a drug for a rare disease is required to file an application for orphan drug.
How to fill out application for orphan drug?
To fill out an application for orphan drug, the sponsor must provide information on the drug's clinical and non-clinical data, the medical condition it treats, and the impact it will have on patients.
What is the purpose of application for orphan drug?
The purpose of an application for orphan drug is to secure regulatory approval and marketing exclusivity for a drug that treats a rare disease, incentivizing pharmaceutical companies to develop treatments for rare conditions.
What information must be reported on application for orphan drug?
The application for orphan drug must include data on the drug's safety, efficacy, and manufacturing process, as well as information on the rare disease it treats, patient population, and unmet medical need.
Fill out your bapplicationb for orphan drug online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Bapplicationb For Orphan Drug is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















