Get the free Technical Guidance on Clinical Evaluation of Medical Devices
Show details
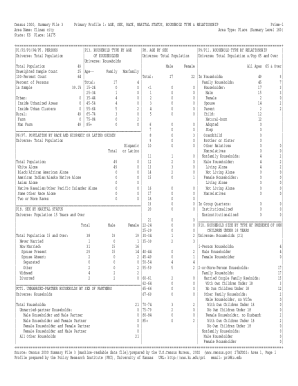
AnnexTechnical Guidance on Clinical Evaluation of Medical Devices
I. Purpose
The clinical evaluation of medical devices is the assessment procedure conducted by
registration applicants to validate
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign technical guidance on clinical
Edit your technical guidance on clinical form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.
Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your technical guidance on clinical form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit technical guidance on clinical online
To use the services of a skilled PDF editor, follow these steps below:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit technical guidance on clinical. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
Dealing with documents is simple using pdfFiller. Now is the time to try it!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out technical guidance on clinical
How to fill out technical guidance on clinical:
01
Start by carefully reading the technical guidance document. Familiarize yourself with its objectives, scope, and requirements.
02
Evaluate the relevance of the technical guidance to your clinical work. Determine if it applies to your specific area of practice or if you need to seek additional guidance tailored to your needs.
03
Identify the key sections and subsections in the technical guidance that require your attention. Pay close attention to any specific instructions, forms to be filled, or data to be provided.
04
Gather all the necessary information and data required to fill out the technical guidance. This may include patient details, test results, medical history, treatment plans, and any other relevant documentation.
05
Ensure that you have a clear understanding of the terminology and terminology used in the technical guidance. If there are any unfamiliar terms or concepts, conduct further research or seek clarification from the appropriate authorities.
06
Organize the information in a logical and structured manner as per the instructions provided in the technical guidance. Use clear and concise language, avoiding jargon or acronyms that may not be universally understood.
07
Be thorough and accurate when filling out the technical guidance. Double-check all the information and cross-reference it with any supporting documentation to ensure consistency and precision.
08
If there are any sections or fields that are not applicable to your situation, make a note and provide a brief explanation or justification as instructed.
09
Review the completed technical guidance form for any errors, omissions, or inconsistencies. Make necessary revisions or clarifications before submitting it to the appropriate authorities or stakeholders.
Who needs technical guidance on clinical:
01
Healthcare professionals: Doctors, nurses, surgeons, and other medical practitioners who provide clinical care to patients can benefit from technical guidance on clinical practices. It helps them stay updated with the latest standards and procedures, ensuring the delivery of safe and effective healthcare services.
02
Clinicians in research and development: Professionals involved in clinical research or development of new medical technologies rely on technical guidance to navigate the regulatory landscape and ensure compliance with ethical guidelines.
03
Health organizations and institutions: Hospitals, clinics, healthcare facilities, and regulatory bodies require technical guidance to establish consistent practices, monitor quality of care, and promote patient safety. It serves as a reference for designing protocols, conducting audits, and assessing adherence to standards.
04
Medical students and trainees: Aspiring healthcare professionals and trainees often seek technical guidance to learn best practices and gain a comprehensive understanding of clinical procedures. It helps them develop the necessary skills and knowledge required for their future practice.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my technical guidance on clinical in Gmail?
technical guidance on clinical and other documents can be changed, filled out, and signed right in your Gmail inbox. You can use pdfFiller's add-on to do this, as well as other things. When you go to Google Workspace, you can find pdfFiller for Gmail. You should use the time you spend dealing with your documents and eSignatures for more important things, like going to the gym or going to the dentist.
How can I get technical guidance on clinical?
It's simple using pdfFiller, an online document management tool. Use our huge online form collection (over 25M fillable forms) to quickly discover the technical guidance on clinical. Open it immediately and start altering it with sophisticated capabilities.
How do I edit technical guidance on clinical on an iOS device?
Use the pdfFiller app for iOS to make, edit, and share technical guidance on clinical from your phone. Apple's store will have it up and running in no time. It's possible to get a free trial and choose a subscription plan that fits your needs.
What is technical guidance on clinical?
Technical guidance on clinical refers to the instructions and recommendations provided to healthcare professionals for conducting and managing clinical trials effectively.
Who is required to file technical guidance on clinical?
The sponsor or principal investigator of a clinical trial is typically responsible for filing technical guidance on clinical.
How to fill out technical guidance on clinical?
Technical guidance on clinical can be filled out by providing detailed information about the trial protocol, study objectives, participant eligibility criteria, and other relevant details.
What is the purpose of technical guidance on clinical?
The purpose of technical guidance on clinical is to ensure that clinical trials are conducted in a safe, ethical, and scientifically rigorous manner.
What information must be reported on technical guidance on clinical?
Technical guidance on clinical must include details about the study design, interventions, endpoints, statistical analysis plan, and monitoring procedures.
Fill out your technical guidance on clinical online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Technical Guidance On Clinical is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.