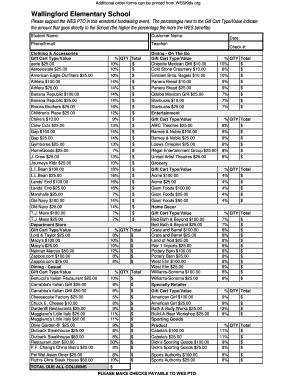
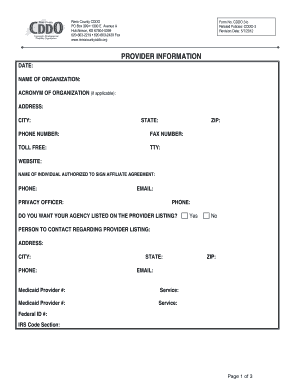
Get the free Adverse events monitoring and reporting guidelines - kznhealth gov
Show details
ADVERSE EVENTS MONITORING AND REPORTING GUIDELINES Compiled by: Dr Okay Mahomes MB CHB (Natal) MBA, FCP HM Department of Public Health Medicine School of Family Medicine and Public Health Nelson Mandela
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign adverse events monitoring and

Edit your adverse events monitoring and form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your adverse events monitoring and form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit adverse events monitoring and online
Follow the steps below to take advantage of the professional PDF editor:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit adverse events monitoring and. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
With pdfFiller, dealing with documents is always straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out adverse events monitoring and

How to fill out adverse events monitoring and:
01
Identify the adverse event: Start by clearly identifying the adverse event that occurred. This could be an unexpected side effect of a medication, an injury, or any other negative outcome related to a product or service.
02
Document the details: Take notes on the specific details of the adverse event. This should include the date and time it occurred, any relevant symptoms or effects experienced, and any factors that may have contributed to the event.
03
Provide contact information: Include your contact information, such as your name, position, and organization, so that you can be easily reached for follow-up or clarification regarding the adverse event.
04
Fill out the adverse events monitoring form: Use the designated adverse events monitoring form provided by your organization or regulatory body. This form will typically require you to provide information about the patient or person affected, any additional medical or treatment details, and a thorough description of the adverse event.
05
Submit the report: Once you have completed the adverse events monitoring form, submit it according to the established procedures within your organization or regulatory body. This may involve submitting it online, mailing it, or handing it over in person.
Who needs adverse events monitoring and:
01
Healthcare professionals: Healthcare professionals, including doctors, nurses, pharmacists, and other medical practitioners, need adverse events monitoring to ensure patient safety and to identify any potential issues or side effects arising from medical treatments or procedures.
02
Pharmaceutical companies: Pharmaceutical companies need adverse events monitoring to identify and assess any potential risks or side effects associated with their products. This allows them to take appropriate actions such as updating warnings, providing additional instructions, or even recalling a product if necessary.
03
Regulatory bodies: Regulatory bodies responsible for the oversight of medical devices, drugs, or other products require adverse events monitoring to assess the safety and effectiveness of these products. This helps them make informed decisions about product approvals, labeling requirements, and post-market surveillance.
04
Consumers and patients: Consumers and patients can also benefit from adverse events monitoring. By reporting any adverse events they experience, they contribute to the ongoing surveillance and monitoring of product safety, which helps protect others and ensures that appropriate actions are taken when necessary.
Overall, adverse events monitoring is crucial for various stakeholders involved in healthcare and product safety to identify, assess, and address any potential risks or issues related to adverse events.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is adverse events monitoring and?
Adverse events monitoring is the process of tracking and reviewing any negative or unexpected events that occur during a medical treatment or with the use of a particular drug or device.
Who is required to file adverse events monitoring and?
Healthcare professionals, pharmaceutical companies, and device manufacturers are required to file adverse events monitoring reports.
How to fill out adverse events monitoring and?
Adverse events monitoring reports can typically be filled out online through specific reporting portals provided by regulatory authorities.
What is the purpose of adverse events monitoring and?
The purpose of adverse events monitoring is to ensure the safety and effectiveness of medical treatments, drugs, and devices by identifying and addressing any potential risks or side effects.
What information must be reported on adverse events monitoring and?
Information such as the type of adverse event, date of occurrence, patient demographics, and details of the medical treatment or product involved must be reported on adverse events monitoring.
How can I manage my adverse events monitoring and directly from Gmail?
Using pdfFiller's Gmail add-on, you can edit, fill out, and sign your adverse events monitoring and and other papers directly in your email. You may get it through Google Workspace Marketplace. Make better use of your time by handling your papers and eSignatures.
How do I make changes in adverse events monitoring and?
With pdfFiller, you may not only alter the content but also rearrange the pages. Upload your adverse events monitoring and and modify it with a few clicks. The editor lets you add photos, sticky notes, text boxes, and more to PDFs.
How do I fill out the adverse events monitoring and form on my smartphone?
Use the pdfFiller mobile app to complete and sign adverse events monitoring and on your mobile device. Visit our web page (https://edit-pdf-ios-android.pdffiller.com/) to learn more about our mobile applications, the capabilities you’ll have access to, and the steps to take to get up and running.
Fill out your adverse events monitoring and online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Adverse Events Monitoring And is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.