Get the free Generic Labelling Requirements of
Show details
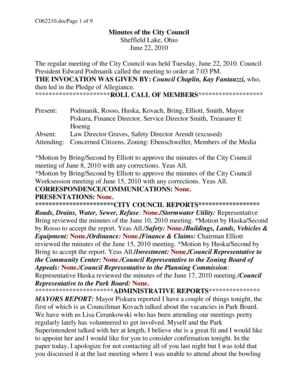
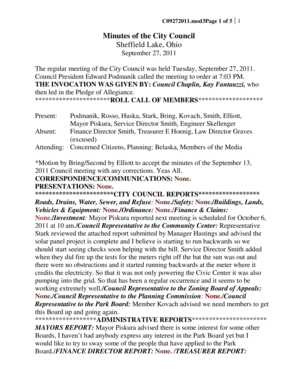
Republic of the Philippines
Department of Health
Food and Drug AdministrationDraft Administrative Order
Revised Rules and Regulations Governing the
Generic Labelling Requirements of
Pharmaceutical
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign generic labelling requirements of

Edit your generic labelling requirements of form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your generic labelling requirements of form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit generic labelling requirements of online
To use our professional PDF editor, follow these steps:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit generic labelling requirements of. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out generic labelling requirements of

Point by Point guide on how to fill out generic labelling requirements:
01
Start by reviewing the applicable regulations: Before beginning the labelling process, it is crucial to familiarize yourself with the regulations and guidelines set by your specific industry or jurisdiction. This will ensure that you meet all the mandatory requirements for labelling.
02
Determine the information to include: Evaluate the necessary information that needs to be included on the label. This generally includes the product's name, ingredients, allergens, nutritional information, manufacturer details, storage instructions, and any required certifications or warnings.
03
Organize the information layout: Plan the layout of the label to ensure all the required information is visible and legible. Consider the font size, color contrast, and readability factors to enhance clarity for the consumer.
04
Use clear and concise language: When providing information on the label, use simple and easily understandable language. Avoid using technical jargon or complicated terms that may confuse the consumer.
05
Accurate measurements and units: Ensure that all measurements and units provided on the label are accurate and comply with relevant standards. For example, if mentioning weight or volume, use the correct units of measurement (grams, milliliters, etc.) and provide accurate numerical values.
06
Include all necessary warnings and precautions: Depending on the nature of the product, it may need to include specific warnings or safety precautions. This is particularly important for products that may pose health risks or require special handling. Prioritize the inclusion of any mandatory warnings to ensure consumer safety.
07
Test readability and legibility: Before finalizing the label, test its readability and legibility. Ensure that the information can be easily read and understood, even in varying lighting conditions or font sizes. This is crucial to prevent any misunderstandings or misinterpretations.
Who needs generic labelling requirements:
01
Manufacturers: Manufacturers of various products, including food, cosmetics, pharmaceuticals, and consumer goods, need to comply with generic labelling requirements. It is essential for them to provide accurate information about their products to ensure consumer safety and regulatory compliance.
02
Retailers: Retailers selling products are responsible for ensuring that the products they sell comply with labelling requirements. They need to verify that the labels on the products they stock accurately represent the necessary information and adhere to applicable regulations.
03
Consumers: While not directly responsible for labelling, consumers benefit from generic labelling requirements. These requirements ensure that consumers have access to essential information about the products they purchase, including ingredients and potential allergens. It enables them to make informed choices and safeguards their well-being.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is generic labelling requirements of?
Generic labelling requirements refer to the standardized information that must be included on labels of products.
Who is required to file generic labelling requirements of?
Manufacturers, distributors, and retailers are required to file generic labelling requirements.
How to fill out generic labelling requirements of?
Generic labelling requirements can be filled out by including all the mandatory information on the product label.
What is the purpose of generic labelling requirements of?
The purpose of generic labelling requirements is to provide consumers with important information about the product and to ensure compliance with regulations.
What information must be reported on generic labelling requirements of?
Information such as product name, ingredients, net weight, expiration date, and manufacturer information must be reported on generic labelling requirements.
Can I create an eSignature for the generic labelling requirements of in Gmail?
You may quickly make your eSignature using pdfFiller and then eSign your generic labelling requirements of right from your mailbox using pdfFiller's Gmail add-on. Please keep in mind that in order to preserve your signatures and signed papers, you must first create an account.
Can I edit generic labelling requirements of on an Android device?
With the pdfFiller mobile app for Android, you may make modifications to PDF files such as generic labelling requirements of. Documents may be edited, signed, and sent directly from your mobile device. Install the app and you'll be able to manage your documents from anywhere.
How do I complete generic labelling requirements of on an Android device?
Use the pdfFiller mobile app and complete your generic labelling requirements of and other documents on your Android device. The app provides you with all essential document management features, such as editing content, eSigning, annotating, sharing files, etc. You will have access to your documents at any time, as long as there is an internet connection.
Fill out your generic labelling requirements of online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Generic Labelling Requirements Of is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.