Get the free Serious Adverse Events (SAE) FollowupFinal Report
Show details
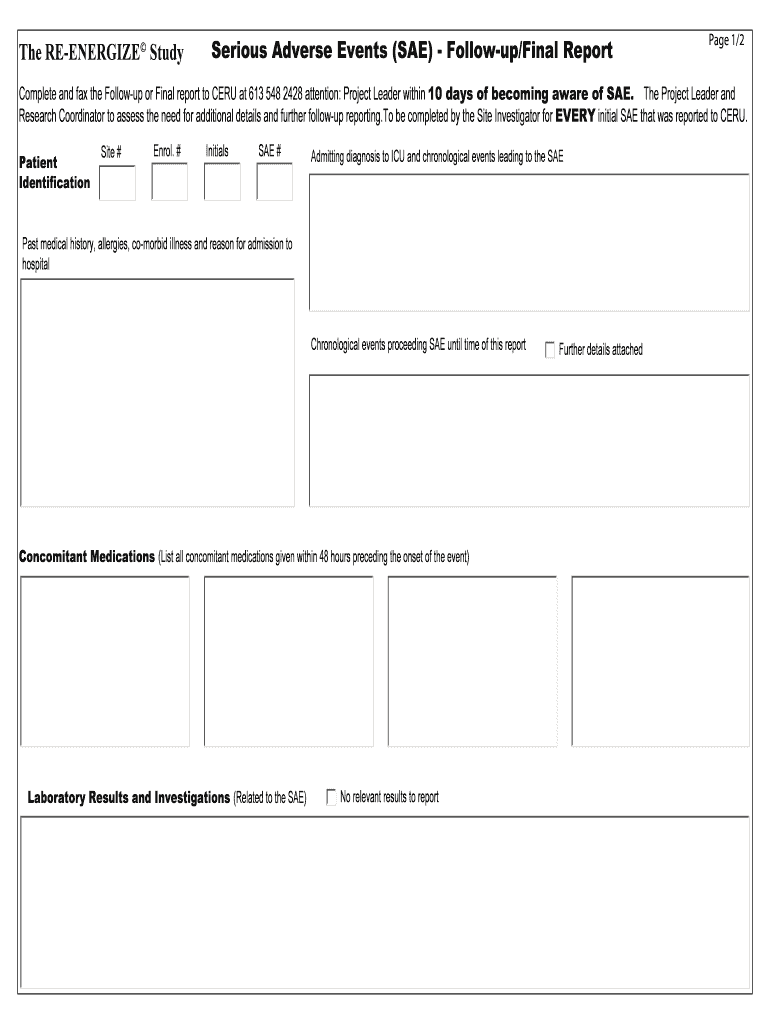
The ENERGIZE Study Serious Adverse Events (SAE) Followup/Final Report Page 1/2 Complete and fax the Followup or Final report to PERU at 613 548 2428 attention: Project Leader within 10 days of becoming
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign serious adverse events sae

Edit your serious adverse events sae form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your serious adverse events sae form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit serious adverse events sae online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit serious adverse events sae. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
With pdfFiller, dealing with documents is always straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out serious adverse events sae

How to Fill out Serious Adverse Events (SAE):
01
Obtain the necessary forms: Start by obtaining the specific Serious Adverse Events (SAE) form required by your organization or regulatory body. This form will typically include sections for documenting important information related to the adverse event.
02
Gather relevant information: Before filling out the SAE form, make sure you have all the necessary information available, such as the participant's identification details, study protocol, medical history, and any relevant laboratory or test results.
03
Describe the adverse event: In the SAE form, provide a detailed description of the adverse event, including the date and time it occurred, the symptoms experienced by the participant, and any other relevant information that helps characterize the event accurately.
04
Assess the event's severity: Evaluate the severity of the adverse event and categorize it according to the criteria provided in the SAE form. Severity levels may range from mild to moderate, severe, or life-threatening. Ensure that your classification aligns with the guidelines provided.
05
Determine the causality: Determine whether the adverse event is related to the studied intervention or other factors. Assess the likelihood of the event being causally related to the intervention by considering factors such as timing, known side effects, past medical history, concurrent illnesses, and any other relevant information.
06
Report concomitant medications: If the participant is taking any other medications or undergoing additional treatments that might contribute to the adverse event, list these concomitant medications in the appropriate section of the SAE form. Include detailed information such as the dosage, frequency, and start and end dates.
07
Record required follow-up actions: Document any actions taken or recommended in response to the adverse event. This may include modifying the study protocol, stopping or adjusting the intervention, initiating additional treatments, or notifying relevant parties such as the principal investigator or ethics committee.
Who needs Serious Adverse Events (SAE)?
01
Researchers and Clinical Trial Investigators: Researchers conducting clinical trials or studies involving experimental drugs, medical devices, or other interventions need to document and report serious adverse events. This helps monitor participant safety, evaluate the intervention's potential risks and benefits, and comply with ethical and regulatory requirements.
02
Ethics Committees and Regulatory Authorities: Ethics committees and regulatory authorities require SAE reports to review the safety and efficacy of interventions. These reports contribute to ongoing monitoring, assessing the study's risk-benefit profile, and providing oversight to ensure participants' welfare.
03
Sponsors and Pharmaceutical Companies: Sponsors and pharmaceutical companies investing in clinical research need to track and report serious adverse events accurately. This information enables them to evaluate the intervention's safety profile, make informed decisions regarding further development or marketing approvals, and fulfill regulatory obligations.
In conclusion, filling out Serious Adverse Events (SAE) forms requires careful attention to detail, accurate documentation of the adverse events, assessing severity and causality, and reporting any required follow-up actions promptly. The individuals responsible for completing SAE forms include researchers, clinical trial investigators, ethics committees, regulatory authorities, sponsors, and pharmaceutical companies involved in the study or development of interventions.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my serious adverse events sae in Gmail?
pdfFiller’s add-on for Gmail enables you to create, edit, fill out and eSign your serious adverse events sae and any other documents you receive right in your inbox. Visit Google Workspace Marketplace and install pdfFiller for Gmail. Get rid of time-consuming steps and manage your documents and eSignatures effortlessly.
How can I modify serious adverse events sae without leaving Google Drive?
People who need to keep track of documents and fill out forms quickly can connect PDF Filler to their Google Docs account. This means that they can make, edit, and sign documents right from their Google Drive. Make your serious adverse events sae into a fillable form that you can manage and sign from any internet-connected device with this add-on.
How do I make changes in serious adverse events sae?
pdfFiller not only allows you to edit the content of your files but fully rearrange them by changing the number and sequence of pages. Upload your serious adverse events sae to the editor and make any required adjustments in a couple of clicks. The editor enables you to blackout, type, and erase text in PDFs, add images, sticky notes and text boxes, and much more.
What is serious adverse events sae?
Serious Adverse Events (SAE) are any untoward medical occurrences that result in death, are life-threatening, require hospitalization or prolongation of existing hospitalization, result in persistent or significant disability or incapacity, or result in a congenital anomaly or birth defect.
Who is required to file serious adverse events sae?
The entities required to file Serious Adverse Events (SAE) include sponsors of clinical trials, investigators, contract research organizations (CROs), and institutional review boards (IRBs).
How to fill out serious adverse events sae?
Serious Adverse Events (SAE) must be filled out by including all relevant information such as the nature of the event, date of occurrence, severity, relationship to the investigational product, and actions taken.
What is the purpose of serious adverse events sae?
The purpose of reporting Serious Adverse Events (SAE) is to ensure the safety of participants in clinical trials, monitor the impact of investigational products on health, and contribute to the overall assessment of a product's safety profile.
What information must be reported on serious adverse events sae?
Information that must be reported on Serious Adverse Events (SAE) includes the description of the event, date of onset, severity, relationship to the investigational product, and outcome.
Fill out your serious adverse events sae online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Serious Adverse Events Sae is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















