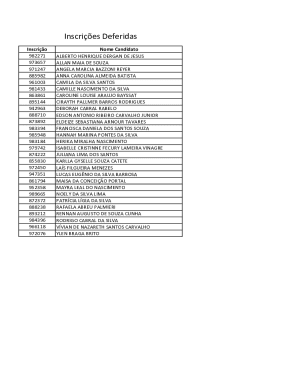
Get the free Chemical Bonds Lewis Dot Structures Worksheetdocx - acschools
Show details
Name Pd Date Chemistry: Chemical Bonds & Lewis Dot Structures Worksheet Intro to Chemical Bonding 1. Why do elements combine to form chemical compounds? 2. Compare and contrast ionic and covalent
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign chemical bonds lewis dot

Edit your chemical bonds lewis dot form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your chemical bonds lewis dot form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing chemical bonds lewis dot online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Check your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit chemical bonds lewis dot. Rearrange and rotate pages, insert new and alter existing texts, add new objects, and take advantage of other helpful tools. Click Done to apply changes and return to your Dashboard. Go to the Documents tab to access merging, splitting, locking, or unlocking functions.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out chemical bonds lewis dot

How to fill out chemical bonds Lewis dot:
01
Identify the atoms involved in the chemical bond. Lewis dot structures are used to represent the valence electrons of an atom in a molecule.
02
Determine the total number of valence electrons for each atom. This can be done by referring to the periodic table and counting the number of electrons in the outermost energy level.
03
Decide which atom will be the central atom in the Lewis dot structure. The central atom is typically the least electronegative and can form multiple bonds with other atoms.
04
Place one pair of electrons between the central atom and each surrounding atom to form single bonds. This will help complete the octets of the outer atoms.
05
Distribute the remaining electrons around the atoms, starting with the outer atoms and then the central atom. Each atom (except hydrogen) should strive to have an octet (8 valence electrons) or a duet (2 valence electrons).
06
If there are still unpaired electrons remaining on the central atom, consider forming double or triple bonds with the surrounding atoms to achieve an octet for the central atom as well.
07
Check if the Lewis dot structure is balanced, meaning all atoms have a full octet or duet around them. Make adjustments if necessary by redistributing the electrons.
08
Ensure the total number of electrons used in the structure is equal to the total number of valence electrons for all atoms involved.
09
The completed Lewis dot structure represents the arrangement of atoms and their valence electrons in a molecule.
Who needs chemical bonds Lewis dot?
01
Chemistry students and educators often use Lewis dot structures to understand and visualize the bonding patterns in molecules.
02
Chemists and researchers rely on Lewis dot structures to predict the reactivity and properties of different compounds.
03
Professionals in fields such as pharmaceuticals, materials science, and chemical engineering may use Lewis dot structures to design and analyze new molecules and materials.
04
Those studying organic chemistry and biochemistry frequently use Lewis dot structures to depict the bonding patterns in organic compounds and biomolecules.
05
Researchers working in fields like quantum chemistry and computational chemistry utilize Lewis dot structures as a starting point for more complex calculations and simulations.
06
Individuals involved in chemical reactions, such as industrial chemists and technicians, can benefit from understanding Lewis dot structures to determine the impact of various reactants and products.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send chemical bonds lewis dot to be eSigned by others?
Once you are ready to share your chemical bonds lewis dot, you can easily send it to others and get the eSigned document back just as quickly. Share your PDF by email, fax, text message, or USPS mail, or notarize it online. You can do all of this without ever leaving your account.
How do I make changes in chemical bonds lewis dot?
The editing procedure is simple with pdfFiller. Open your chemical bonds lewis dot in the editor. You may also add photos, draw arrows and lines, insert sticky notes and text boxes, and more.
How do I fill out chemical bonds lewis dot using my mobile device?
On your mobile device, use the pdfFiller mobile app to complete and sign chemical bonds lewis dot. Visit our website (https://edit-pdf-ios-android.pdffiller.com/) to discover more about our mobile applications, the features you'll have access to, and how to get started.
What is chemical bonds lewis dot?
Lewis dot structure, also called Lewis structure or electron dot structure, is a diagram showing the bonding between atoms in a molecule.
Who is required to file chemical bonds lewis dot?
Students or individuals studying chemistry are usually required to fill out chemical bonds Lewis dot diagrams.
How to fill out chemical bonds lewis dot?
To fill out a Lewis dot diagram, first determine the total number of valence electrons in the molecule, then distribute the electrons around the atoms to show bonding pairs and lone pairs.
What is the purpose of chemical bonds lewis dot?
The purpose of a Lewis dot structure is to show how electrons are distributed in a molecule and how atoms are bonded together.
What information must be reported on chemical bonds lewis dot?
The Lewis dot diagram must include the symbols of the atoms in the molecule and the arrangement of valence electrons.
Fill out your chemical bonds lewis dot online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Chemical Bonds Lewis Dot is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.