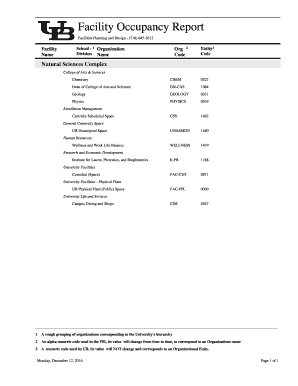
Get the free Phase II Study of Dose-Adjusted EPOCH- in Adults - kccop
Show details
Version Date: 09/03/2014 NCI CC Protocol #:10C0052 J NCI STEP protocol #: 9177 Phase II Study of Readjusted EPOCH+/ in Adults With Untreated Burkitt Lymphoma, CMC Positive Diffuse Large Cell Lymphoma
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign phase ii study of

Edit your phase ii study of form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your phase ii study of form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing phase ii study of online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit phase ii study of. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out phase ii study of

How to fill out phase II study of:
01
Gather all necessary information: Start by collecting all relevant documents, protocols, and guidelines related to the phase II study. Familiarize yourself with the study objectives, design, and any specific criteria or procedures that need to be followed.
02
Review previous phase I study findings: It is essential to understand the background and results of any previous phase I study conducted, as it sets the foundation for the phase II study. Analyze the data obtained in phase I to identify potential safety concerns, dosing regimens, and endpoints for the phase II study.
03
Define study population and eligibility criteria: Clearly specify the target population for the phase II study. Determine the inclusion and exclusion criteria that participants need to meet to be eligible for enrollment. This helps ensure the study results are representative of the intended patient population.
04
Develop a comprehensive study protocol: Create a detailed study protocol that outlines the objectives, methodology, study endpoints, timeline, and statistical analysis plan. Consider incorporating any modifications or improvements based on the lessons learned from previous studies or ongoing research.
05
Obtain necessary approvals: Before initiating the phase II study, ensure that you have obtained all required approvals from relevant ethics committees, institutional review boards, and regulatory authorities. Comply with all local and international regulations to protect the rights and well-being of study participants.
06
Recruit and enroll study participants: Implement a comprehensive recruitment strategy to identify and enroll eligible participants. Advertise the study through appropriate channels, collaborate with clinical sites, and ensure informed consent is obtained from each participant before their inclusion in the study.
07
Conduct study interventions and data collection: Follow the study protocol to administer the investigational treatment or intervention to participants. Collect data on efficacy measures, safety outcomes, and any other relevant parameters based on the study design. Ensure proper documentation and accurate record-keeping of all study procedures.
08
Monitor and manage the study: Continuously monitor the progress of the phase II study, including adherence to the protocol, participant enrollment rates, and data quality. Implement strategies to address any challenges or issues that may arise during the study period. Collaborate with study sites and investigators to ensure proper study conduct.
09
Analyze and interpret study results: Once all data collection is complete, perform a comprehensive analysis of the study results. Use appropriate statistical methods to interpret the data and assess the primary and secondary endpoints. Compare the findings with predefined success criteria to determine the efficacy and safety of the investigational product or intervention.
10
Prepare study report and disseminate findings: Summarize the study results, including significant findings, limitations, and implications, into a comprehensive study report. Share the report with key stakeholders, such as study sponsors, regulatory authorities, and scientific communities to contribute to the overall knowledge in the field.
Who needs phase II study of:
01
Pharmaceutical and biotechnology companies: These companies conduct phase II studies to assess the safety and efficacy of their new drugs or interventions before advancing them to larger-scale trials. Phase II studies provide critical information on the potential benefits and risks associated with the investigational product.
02
Researchers and scientists: Researchers and scientists involved in drug discovery and development rely on phase II studies to understand the effects of experimental treatments on specific diseases or conditions. The results obtained from these studies help guide further research and decision-making processes.
03
Regulatory authorities: Regulatory authorities, such as the Food and Drug Administration (FDA) in the United States, require phase II study data as part of the regulatory approval process. These authorities carefully assess the study results to determine whether the investigational product should progress to phase III trials or be granted marketing authorization.
04
Healthcare professionals: Physicians, nurses, and other healthcare professionals rely on phase II study findings to gain insights into the potential benefits and risks of experimental treatments. This information helps them make informed decisions regarding the appropriate use of these interventions in clinical practice.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is phase ii study of?
Phase II study is a clinical trial that evaluates the effectiveness and safety of a medical treatment or intervention in a larger group of patients.
Who is required to file phase ii study of?
The pharmaceutical company or research institution conducting the study is required to file the Phase II study.
How to fill out phase ii study of?
The Phase II study is filled out by providing detailed information about the study protocol, participant demographics, treatment regimen, and outcomes.
What is the purpose of phase ii study of?
The purpose of Phase II study is to further investigate the safety and efficacy of a treatment identified in Phase I clinical trials.
What information must be reported on phase ii study of?
Information such as study design, patient enrollment criteria, treatment regimen, adverse events, and study outcomes must be reported on the Phase II study.
Can I sign the phase ii study of electronically in Chrome?
You certainly can. You get not just a feature-rich PDF editor and fillable form builder with pdfFiller, but also a robust e-signature solution that you can add right to your Chrome browser. You may use our addon to produce a legally enforceable eSignature by typing, sketching, or photographing your signature with your webcam. Choose your preferred method and eSign your phase ii study of in minutes.
Can I create an electronic signature for signing my phase ii study of in Gmail?
It's easy to make your eSignature with pdfFiller, and then you can sign your phase ii study of right from your Gmail inbox with the help of pdfFiller's add-on for Gmail. This is a very important point: You must sign up for an account so that you can save your signatures and signed documents.
How do I edit phase ii study of straight from my smartphone?
The best way to make changes to documents on a mobile device is to use pdfFiller's apps for iOS and Android. You may get them from the Apple Store and Google Play. Learn more about the apps here. To start editing phase ii study of, you need to install and log in to the app.
Fill out your phase ii study of online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Phase Ii Study Of is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.