Get the free student exploration titration
Show details
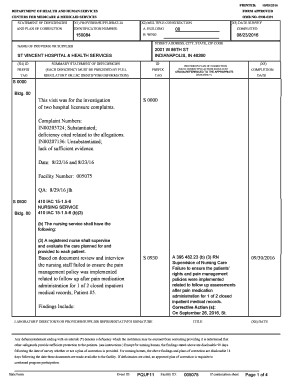
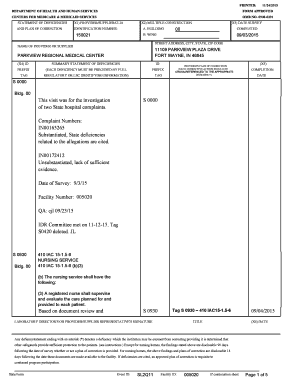
Name Student Exploration Titration Go to www. explorelearning. com. If you have not created a login yet use the code provided on the Promethean board. Vocabulary acid analyte base dissociate equivalence point indicator litmus paper molarity neutralize pH strong acid strong base titrant titration titration curve weak acid weak base Gizmo Warm-up Litmus is an example of an indicator a substance that changes color depending on its pH pH is a measure of the concentration of protons or H3O 1 ions....
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign student exploration titration

Edit your student exploration titration form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your student exploration titration form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing student exploration titration online
Use the instructions below to start using our professional PDF editor:
1
Log in to account. Start Free Trial and sign up a profile if you don't have one.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit student exploration titration. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out student exploration titration

How to Fill Out Student Exploration Titration:
01
Begin by gathering all necessary materials for the titration experiment. This typically includes a burette, a standardized solution, a flask, an indicator, and the substance being titrated.
02
Set up the laboratory workspace by securing the burette in a burette stand and placing the flask underneath it. Ensure that the workspace is clean and organized to prevent any contamination.
03
Prepare the standardized solution by carefully measuring the appropriate volume into the burette. Take note of the initial volume.
04
Add a few drops of the indicator to the substance being titrated in the flask. The indicator will change color at the endpoint of the titration.
05
Slowly release the standardized solution from the burette into the flask while stirring continuously. Monitor the color change of the indicator. Once the color change becomes permanent, the endpoint has been reached.
06
Record the final volume of the standardized solution in the burette. This is known as the volume of the titrant used in the titration.
07
Repeat the titration process if necessary to ensure accuracy and obtain consistent results. Calculate the average volume of the standardized solution used.
08
Use the volumes and concentrations of the substances involved to calculate the concentration of the unknown solution being titrated. This can be done using the stoichiometry of the balanced chemical equation.
Who Needs Student Exploration Titration:
01
Chemistry students studying the principles of titration and acid-base reactions benefit from conducting student exploration titrations. It allows them to apply theoretical knowledge to practical experiments and gain hands-on experience.
02
Students pursuing degrees or courses in analytical chemistry or laboratory techniques often use student exploration titration as a fundamental skill. It helps them develop proficiency in accurate measurement, observation, and data analysis.
03
Researchers and scientists who need to determine the concentration of substances in solutions rely on titration techniques. Student exploration titration provides a basis for understanding and performing more advanced and specialized titration methods in professional laboratory settings.
In summary, the process of filling out a student exploration titration involves gathering materials, setting up the workspace, preparing the standardized solution, adding the indicator, gradually releasing the solution, recording volumes, and calculating concentrations. This technique is essential for students studying chemistry, as well as professionals in research and analytical fields.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make edits in student exploration titration without leaving Chrome?
student exploration titration can be edited, filled out, and signed with the pdfFiller Google Chrome Extension. You can open the editor right from a Google search page with just one click. Fillable documents can be done on any web-connected device without leaving Chrome.
How do I fill out the student exploration titration form on my smartphone?
Use the pdfFiller mobile app to fill out and sign student exploration titration on your phone or tablet. Visit our website to learn more about our mobile apps, how they work, and how to get started.
Can I edit student exploration titration on an iOS device?
Create, edit, and share student exploration titration from your iOS smartphone with the pdfFiller mobile app. Installing it from the Apple Store takes only a few seconds. You may take advantage of a free trial and select a subscription that meets your needs.
What is student exploration titration?
Student exploration titration is a laboratory technique used to determine the concentration of a solution by gradually adding a solution of known concentration until a reaction is completed.
Who is required to file student exploration titration?
Students conducting experiments in chemistry or related fields are required to perform and report on student exploration titration.
How to fill out student exploration titration?
To fill out student exploration titration, students must carefully follow the experimental procedure, record their measurements accurately, and calculate the results based on the titration curve.
What is the purpose of student exploration titration?
The purpose of student exploration titration is to teach students how to accurately measure the concentration of a solution and understand the principles of stoichiometry in chemical reactions.
What information must be reported on student exploration titration?
Students must report the initial and final volumes of the titrant, the molarity of the titrant, the volume of the analyte, and the calculated concentration of the analyte.
Fill out your student exploration titration online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Student Exploration Titration is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.