Get the free MOLE Practice Problems
Show details
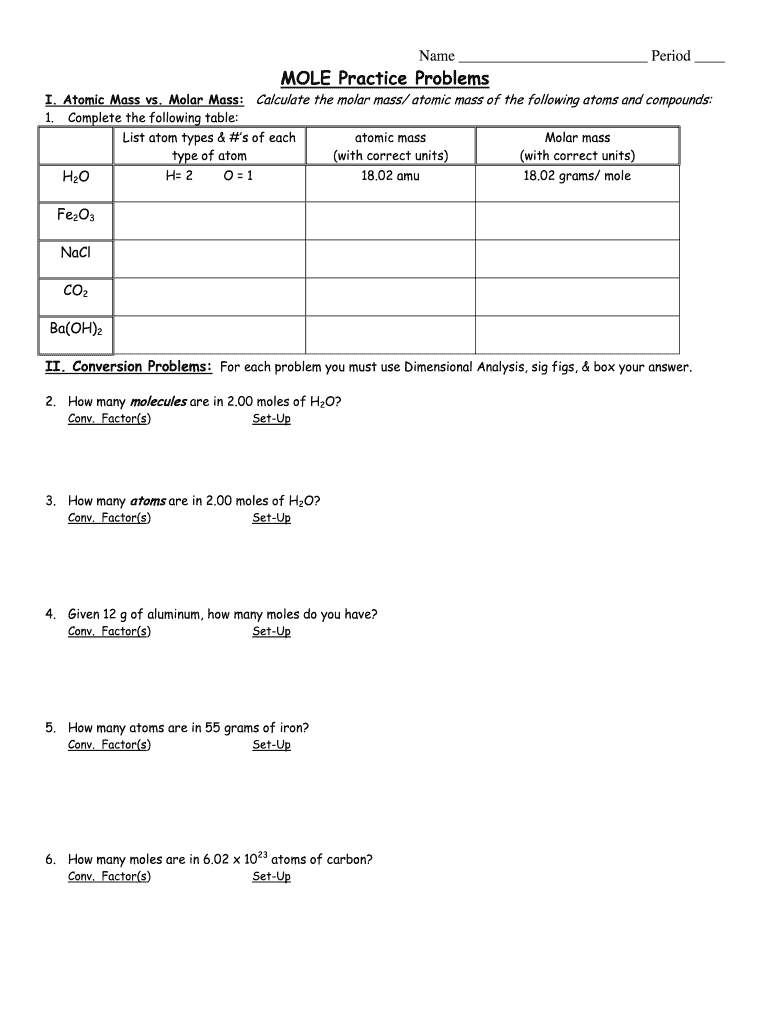
Name Period MOLE Practice Problems. Atomic Mass vs. Molar Mass: Calculate the molar mass/ atomic mass of the following atoms and compounds: 1. Complete the following table: List atom types & #s of
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign mole practice problems

Edit your mole practice problems form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your mole practice problems form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing mole practice problems online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit mole practice problems. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out mole practice problems

How to fill out mole practice problems:
01
Understand the concept of molar mass: Before starting with mole practice problems, it is essential to understand the concept of molar mass. Molar mass refers to the mass of one mole of a substance and is expressed in grams per mole (g/mol). To determine the molar mass of a compound, add up the atomic masses of all the atoms present in the compound.
02
Convert given quantities to moles: The next step is to convert the given quantities into moles. This can be achieved by dividing the given mass or number of particles by the molar mass of the substance. For example, if given the mass of a substance is 50 grams, and its molar mass is 25 g/mol, then the number of moles would be 50 g / 25 g/mol = 2 mol.
03
Use the mole ratio to solve problems: Mole ratio refers to the ratio of moles of one substance to moles of another substance in a balanced chemical equation. It is crucial in solving mole practice problems as it allows you to convert between different substances. By applying the mole ratio, you can determine the moles of one substance based on the known moles of another substance.
04
Apply the concept of Avogadro's number: Avogadro's number, also known as the mole constant, is a fundamental value in chemistry that represents the number of atoms, molecules, or particles in one mole of a substance. It is approximately equal to 6.022 x 10^23. It helps in converting between moles and particles or vice versa. To convert from moles to particles, multiply the number of moles by Avogadro's number. For example, if you have 2 moles of a substance, the number of particles would be 2 mol x 6.022 x 10^23 = 1.2044 x 10^24 particles.
Who needs mole practice problems:
01
Chemistry students: Mole practice problems are particularly useful for chemistry students who are learning about stoichiometry, gas laws, and various other topics related to the mole concept. By practicing such problems, students can strengthen their understanding and application of mole calculations.
02
Lab technicians: Lab technicians who work in analytical chemistry or other fields that involve precise measurements often encounter mole calculations. Practice problems can help them improve their skills and accuracy when performing experiments and analyzing data.
03
Professionals in chemical industries: Professionals working in chemical industries, such as pharmaceuticals, petrochemicals, or material sciences, frequently use mole calculations in their work. Having a firm grasp of these calculations can enhance their ability to design and optimize processes, determine quantities of reactants, and analyze product yields.
In summary, understanding how to fill out mole practice problems involves comprehending the concept of molar mass, converting given quantities to moles, using the mole ratio to solve problems, and applying Avogadro's number to convert between moles and particles. Mole practice problems are valuable for chemistry students, lab technicians, and professionals in chemical industries who encounter mole calculations in their studies or work.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit mole practice problems from Google Drive?
It is possible to significantly enhance your document management and form preparation by combining pdfFiller with Google Docs. This will allow you to generate papers, amend them, and sign them straight from your Google Drive. Use the add-on to convert your mole practice problems into a dynamic fillable form that can be managed and signed using any internet-connected device.
How do I edit mole practice problems online?
The editing procedure is simple with pdfFiller. Open your mole practice problems in the editor, which is quite user-friendly. You may use it to blackout, redact, write, and erase text, add photos, draw arrows and lines, set sticky notes and text boxes, and much more.
How do I edit mole practice problems on an iOS device?
Use the pdfFiller app for iOS to make, edit, and share mole practice problems from your phone. Apple's store will have it up and running in no time. It's possible to get a free trial and choose a subscription plan that fits your needs.
What is mole practice problems?
Mole practice problems are chemistry problems that involve calculating the amount of a substance in moles.
Who is required to file mole practice problems?
Students studying chemistry or anyone working with chemical reactions may be required to solve mole practice problems.
How to fill out mole practice problems?
Mole practice problems are typically solved using the formula n = m/M, where n is the number of moles, m is the mass of the substance, and M is the molar mass of the substance.
What is the purpose of mole practice problems?
The purpose of mole practice problems is to help understand and apply the concept of mole and molar mass in chemistry calculations.
What information must be reported on mole practice problems?
Mole practice problems typically require information such as the mass of the substance, the molar mass of the substance, and the number of moles.
Fill out your mole practice problems online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Mole Practice Problems is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















