Get the free Ionic or Covalent Bonding Lab - psd150org
Show details
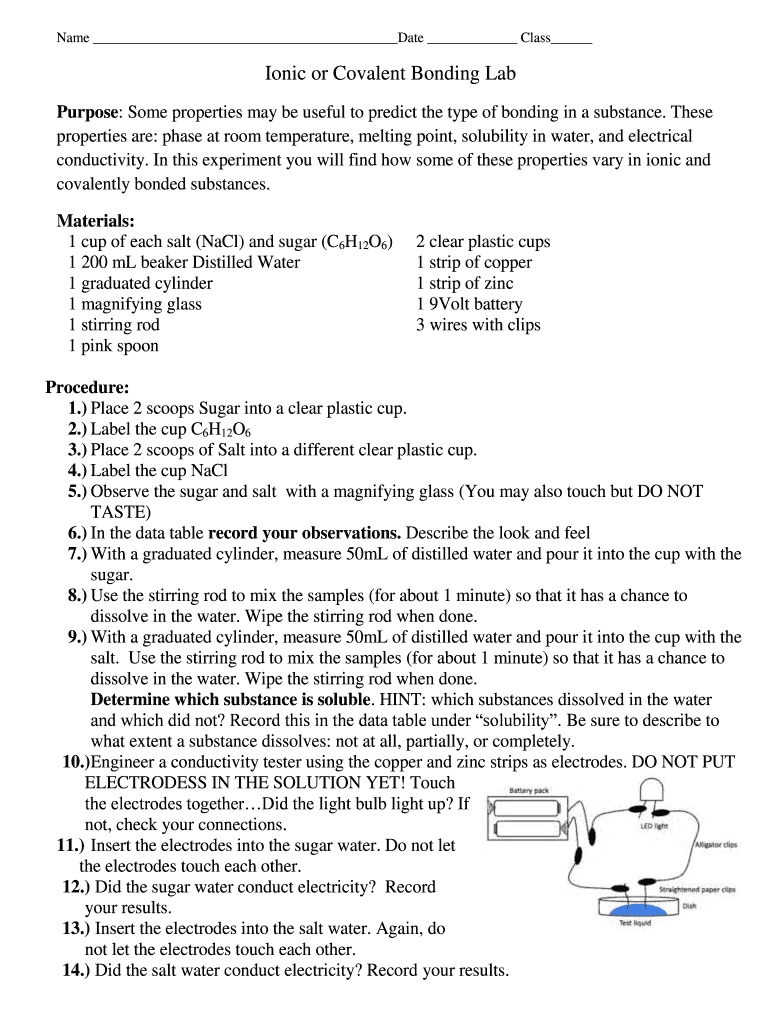
Name Date Class Ionic or Covalent Bonding Lab Purpose: Some properties may be useful to predict the type of bonding in a substance. These properties are: phase at room temperature, melting point,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign ionic or covalent bonding

Edit your ionic or covalent bonding form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your ionic or covalent bonding form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit ionic or covalent bonding online
To use the professional PDF editor, follow these steps:
1
Log in to your account. Click Start Free Trial and register a profile if you don't have one yet.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit ionic or covalent bonding. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out ionic or covalent bonding

How to fill out ionic or covalent bonding:
01
Understand the concept of chemical bonding: Before filling out ionic or covalent bonding, it is important to have a basic understanding of what chemical bonding is. Ionic bonding involves the transfer of electrons between atoms, resulting in the formation of ions, while covalent bonding involves the sharing of electrons between atoms.
02
Identify the elements involved: Determine which elements are participating in the bonding process. This will help determine whether the bonding is likely to be ionic or covalent. Ionic bonding generally occurs between a metal and a non-metal, while covalent bonding typically occurs between two non-metals.
03
Determine the electronegativity difference: Calculate the electronegativity difference between the elements. Electronegativity is a measure of an atom's ability to attract electrons towards itself. If the electronegativity difference is large (greater than 1.7), it indicates that the bonding is likely to be ionic. If the electronegativity difference is small (less than 1.7), it suggests that the bonding is likely to be covalent.
04
Analyze the octet rule: Consider whether the elements involved are likely to follow the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with a full outer shell of electrons. If an element is not likely to follow the octet rule, it may indicate that the bonding is more likely to be covalent.
05
Determine the type of bonding: Based on the previous steps, decide whether the bonding is more likely to be ionic or covalent. If the electronegativity difference is large and the elements involved are following the octet rule, it suggests that the bonding is ionic. If the electronegativity difference is small and the elements involved are not likely to follow the octet rule, it suggests that the bonding is covalent.
Who needs ionic or covalent bonding:
01
Chemists and scientists: Chemists and scientists studying the properties and interactions of different substances rely on understanding ionic and covalent bonding. This knowledge helps them explain the behavior of molecules, predict chemical reactions, and develop new materials.
02
Students studying chemistry: Students learning chemistry at various educational levels need to understand ionic and covalent bonding as it forms the foundation for understanding chemical compounds and reactions. It helps them grasp concepts such as Lewis structures, molecular geometry, and intermolecular forces.
03
Engineers and material scientists: Professionals working in engineering and material science fields require knowledge of ionic and covalent bonding to design and manipulate materials with specific properties. This understanding aids in developing advanced materials for applications in electronics, aerospace, medicine, and many other industries.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my ionic or covalent bonding directly from Gmail?
The pdfFiller Gmail add-on lets you create, modify, fill out, and sign ionic or covalent bonding and other documents directly in your email. Click here to get pdfFiller for Gmail. Eliminate tedious procedures and handle papers and eSignatures easily.
How do I edit ionic or covalent bonding straight from my smartphone?
The pdfFiller apps for iOS and Android smartphones are available in the Apple Store and Google Play Store. You may also get the program at https://edit-pdf-ios-android.pdffiller.com/. Open the web app, sign in, and start editing ionic or covalent bonding.
Can I edit ionic or covalent bonding on an Android device?
With the pdfFiller Android app, you can edit, sign, and share ionic or covalent bonding on your mobile device from any place. All you need is an internet connection to do this. Keep your documents in order from anywhere with the help of the app!
What is ionic or covalent bonding?
Ionic bonding is the complete transfer of valence electron(s) between atoms, while covalent bonding is the sharing of pairs of electrons between atoms.
Who is required to file ionic or covalent bonding?
Chemists, researchers, and students studying chemistry are required to understand and work with ionic and covalent bonding.
How to fill out ionic or covalent bonding?
To fill out ionic or covalent bonding, one must understand the properties of the elements involved, determine their valence electrons, and follow the rules for forming bonds.
What is the purpose of ionic or covalent bonding?
The purpose of ionic and covalent bonding is to form stable compounds by achieving a full outer electron shell, leading to lower energy levels for the atoms involved.
What information must be reported on ionic or covalent bonding?
Information such as the types of atoms involved, the number of valence electrons, the bond type (ionic or covalent), and the resulting compound formed must be reported on ionic or covalent bonding.
Fill out your ionic or covalent bonding online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Ionic Or Covalent Bonding is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















