Get the free CLINICAL DEVELOPMENT GOALS C - College of Education - education uoregon
Show details
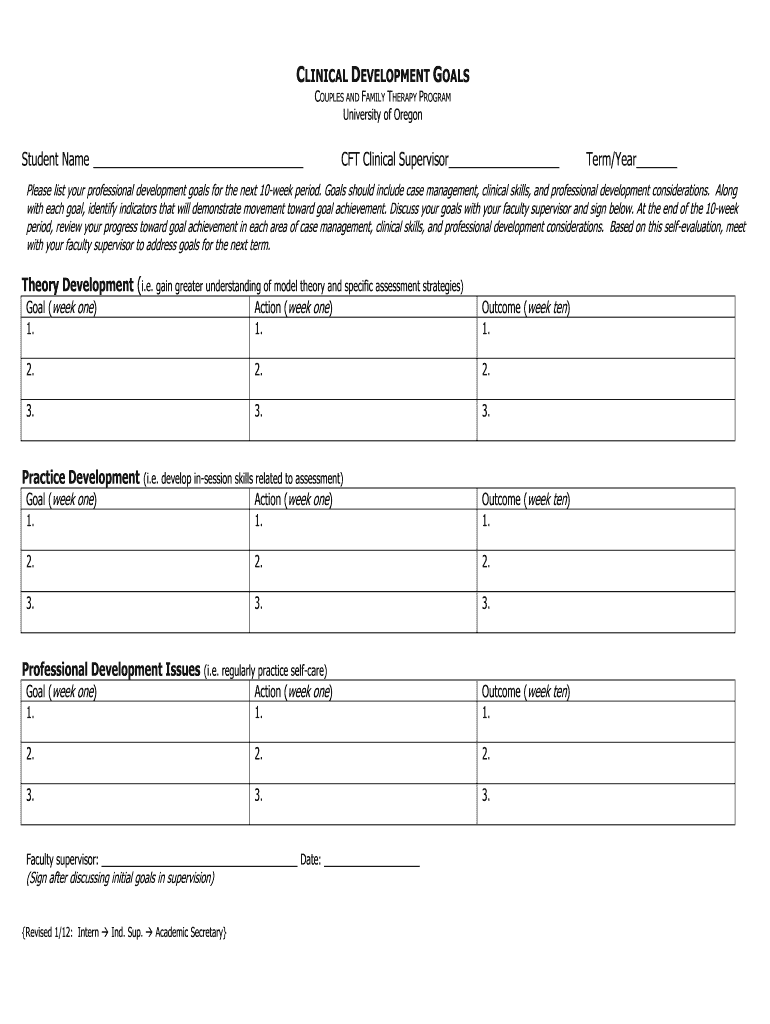
CLINICAL DEVELOPMENT GOALS COUPLES AND FAMILY THERAPY PROGRAM University of Oregon Student Name CFT Clinical Supervisor Term/Year Please list your professional development goals for the next 10week
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical development goals c

Edit your clinical development goals c form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical development goals c form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit clinical development goals c online
To use the professional PDF editor, follow these steps below:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit clinical development goals c. Add and replace text, insert new objects, rearrange pages, add watermarks and page numbers, and more. Click Done when you are finished editing and go to the Documents tab to merge, split, lock or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical development goals c

How to fill out clinical development goals c:
01
Start by clearly defining your objectives: Before filling out the clinical development goals form, it's essential to have a clear understanding of what you want to achieve. Consider the specific outcomes and milestones you aim to reach during the clinical development process.
02
Align with regulatory requirements: As you fill out the clinical development goals form, ensure that your goals comply with relevant regulatory requirements. Be aware of any specific guidelines or standards that govern the development of your specific product or treatment.
03
Consider the timeline and resources: Take into account the time and resources available for your clinical development efforts. This includes factors such as budget, personnel, and equipment. Your goals should be realistic and attainable within the given constraints.
04
Incorporate risk management: Assess potential risks and uncertainties associated with your clinical development goals. Address how you will mitigate these risks and ensure patient safety throughout the process.
05
Collaborate and communicate: Involve key stakeholders, such as researchers, clinicians, and regulatory authorities, in the goal-setting process. Seek input and feedback to ensure that the goals align with the broader objectives of your organization and the needs of patients.
Who needs clinical development goals c:
01
Pharmaceutical companies: Pharmaceutical companies that are engaged in the development of new drugs or therapies require clinical development goals to guide their research activities. These goals help outline the specific targets and objectives they aim to achieve during the clinical trial phases.
02
Research institutions: Academic and research institutions involved in scientific studies and clinical trials also benefit from clinical development goals. These goals provide structure and direction for their research efforts, ensuring that they contribute to the advancement of medical knowledge.
03
Regulatory authorities: Regulatory authorities responsible for overseeing and approving clinical trials often require clinical development goals to assess the progress and viability of the research. These goals help evaluate whether the study is conducted according to predetermined objectives and aligns with regulatory standards.
04
Healthcare professionals: Healthcare professionals involved in clinical research may use clinical development goals as a tool to guide their practice and ensure that they follow the established guidelines during the study. These goals help them focus on specific endpoints and ensure the safety and well-being of study participants.
05
Patients and patient advocacy groups: Clinical development goals are also important for patients and patient advocacy groups. They provide insights into the objectives of the research and help set realistic expectations for potential treatments or therapeutic interventions. Patients can advocate for their needs and contribute to the development of goals that align with their interests and priorities.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Can I create an eSignature for the clinical development goals c in Gmail?
Upload, type, or draw a signature in Gmail with the help of pdfFiller’s add-on. pdfFiller enables you to eSign your clinical development goals c and other documents right in your inbox. Register your account in order to save signed documents and your personal signatures.
How can I fill out clinical development goals c on an iOS device?
Make sure you get and install the pdfFiller iOS app. Next, open the app and log in or set up an account to use all of the solution's editing tools. If you want to open your clinical development goals c, you can upload it from your device or cloud storage, or you can type the document's URL into the box on the right. After you fill in all of the required fields in the document and eSign it, if that is required, you can save or share it with other people.
How do I edit clinical development goals c on an Android device?
You can make any changes to PDF files, such as clinical development goals c, with the help of the pdfFiller mobile app for Android. Edit, sign, and send documents right from your mobile device. Install the app and streamline your document management wherever you are.
What is clinical development goals c?
Clinical development goals c are specific objectives set by a clinical development team to achieve during the course of a clinical trial.
Who is required to file clinical development goals c?
The clinical development team is responsible for setting and filing clinical development goals c.
How to fill out clinical development goals c?
Clinical development goals c can be filled out by outlining specific milestones, timelines, and success criteria for the clinical trial.
What is the purpose of clinical development goals c?
The purpose of clinical development goals c is to provide a roadmap for the successful completion of a clinical trial.
What information must be reported on clinical development goals c?
Information such as trial objectives, patient recruitment targets, data collection timelines, and regulatory compliance measures must be reported on clinical development goals c.
Fill out your clinical development goals c online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Development Goals C is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















