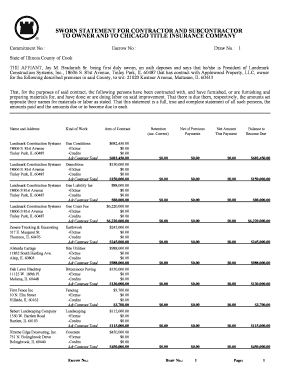
Get the free Investigational Product Transfer bFormb - NIAID - National Institutes of bb - niaid nih
Show details
Instructions to Complete This Form: 1. 2. 3. 4. 5. 6. 7. 8. Before completing and signing this form, refer to the Transfer of Investigational Product section in the Pharmacy Guidelines and Instructions
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign investigational product transfer bformb

Edit your investigational product transfer bformb form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your investigational product transfer bformb form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit investigational product transfer bformb online
To use the services of a skilled PDF editor, follow these steps:
1
Log in. Click Start Free Trial and create a profile if necessary.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit investigational product transfer bformb. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
With pdfFiller, it's always easy to work with documents. Try it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out investigational product transfer bformb

How to fill out investigational product transfer form?
01
Start by obtaining the investigational product transfer form (bFormb) from the relevant authority or organization overseeing the trial or study. This form is usually provided to ensure proper documentation and tracking of the transfer of investigational products between parties.
02
Fill out the sender's information: Provide your name, contact details, and any other required information as the sender of the investigational product.
03
Fill out the receiver's information: Include the name, contact details, and any other necessary information of the receiver who will be handling the investigational product.
04
Provide details about the investigational product: This includes the name of the product, its lot or batch number, and any other identifying information. Ensure accuracy in providing this information to avoid confusion or errors during the transfer.
05
Describe the nature of the transfer: Specify whether it is a shipment, hand delivery, or any other means of transfer. Indicate the date and time of transfer as well.
06
Include any special handling or storage instructions: If there are any specific requirements or precautions involved in the transfer of the investigational product, such as temperature control or storage conditions, make sure to note them in this section.
07
Document the transfer process: Provide step-by-step details of how the transfer will take place, including any packaging, labeling, or shipment details. This helps ensure a smooth and traceable transfer process.
08
Obtain appropriate signatures: Ensure that both the sender and receiver sign and date the form to acknowledge their agreement and responsibility for the transfer. If required, additional approvals or signatures may be needed from relevant parties involved in the trial or study.
Who needs investigational product transfer form?
01
Sponsors and contract research organizations (CROs): These entities are responsible for initiating and overseeing clinical trials or studies. They may need to transfer investigational products to various study sites or other parties involved in the trial.
02
Investigational sites: The sites where clinical trials or studies are conducted may receive investigational products from sponsors or CROs for use in the trial. They need the transfer form to document the receipt and handling of these products.
03
Third-party vendors or distributors: In some cases, third-party vendors or distributors may be involved in the distribution or storage of investigational products. They may require the transfer form to ensure proper documentation and compliance with regulations.
In summary, to fill out the investigational product transfer form (bFormb), provide the necessary information about the sender and receiver, describe the investigational product, specify the transfer nature and process, and obtain appropriate signatures. This form is needed by sponsors, contract research organizations, investigational sites, and third-party vendors/distributors involved in clinical trials or studies.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I manage my investigational product transfer bformb directly from Gmail?
Using pdfFiller's Gmail add-on, you can edit, fill out, and sign your investigational product transfer bformb and other papers directly in your email. You may get it through Google Workspace Marketplace. Make better use of your time by handling your papers and eSignatures.
How do I make changes in investigational product transfer bformb?
With pdfFiller, you may not only alter the content but also rearrange the pages. Upload your investigational product transfer bformb and modify it with a few clicks. The editor lets you add photos, sticky notes, text boxes, and more to PDFs.
How can I edit investigational product transfer bformb on a smartphone?
You may do so effortlessly with pdfFiller's iOS and Android apps, which are available in the Apple Store and Google Play Store, respectively. You may also obtain the program from our website: https://edit-pdf-ios-android.pdffiller.com/. Open the application, sign in, and begin editing investigational product transfer bformb right away.
What is investigational product transfer form?
The investigational product transfer form is a document used to track the transfer of investigational products from one site to another during a clinical trial.
Who is required to file investigational product transfer form?
The sponsor or investigator responsible for the clinical trial is required to file the investigational product transfer form.
How to fill out investigational product transfer form?
The investigational product transfer form should be filled out with details of the transfer including the quantity of product, date of transfer, sender and receiver information, and any special handling instructions.
What is the purpose of investigational product transfer form?
The purpose of the investigational product transfer form is to ensure that the transfer of investigational products is properly documented and tracked to maintain the integrity of the clinical trial.
What information must be reported on investigational product transfer form?
The investigational product transfer form must include details such as the product name, lot number, expiration date, quantity transferred, date of transfer, sender and receiver information, and any special handling instructions.
Fill out your investigational product transfer bformb online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Investigational Product Transfer Bformb is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.