Get the free Planning your Bioscience Regulatory Affairs Concentration rev 2 20110922docx - advan...
Show details
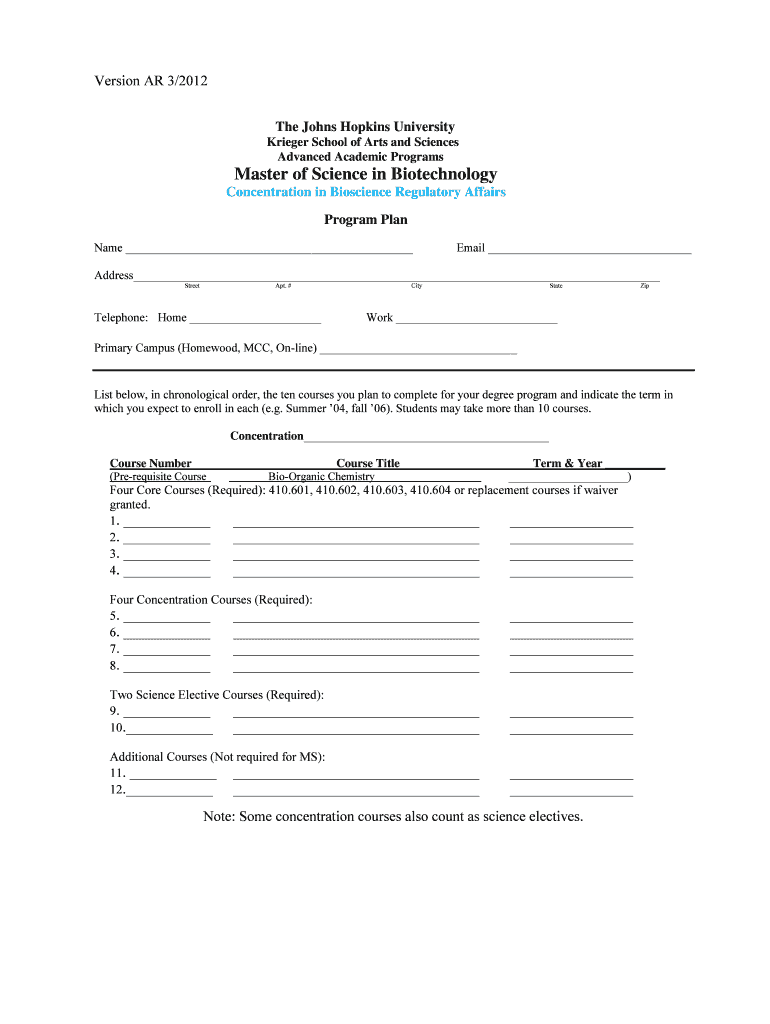
Version AR 3/2012 The Johns Hopkins University Krieger School of Arts and Sciences Advanced Academic Programs Master of Science in Biotechnology Concentration in Bioscience Regulatory Affairs Program
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign planning your bioscience regulatory

Edit your planning your bioscience regulatory form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your planning your bioscience regulatory form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit planning your bioscience regulatory online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit planning your bioscience regulatory. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it from your records list. Then, click the right toolbar and select one of the various exporting options: save in numerous formats, download as PDF, email, or cloud.
It's easier to work with documents with pdfFiller than you could have believed. You can sign up for an account to see for yourself.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out planning your bioscience regulatory

How to Fill Out Planning Your Bioscience Regulatory:
01
Start by gathering all the necessary information and documents related to your bioscience project. This may include research data, product specifications, and any previous regulatory approvals.
02
Identify the specific regulatory requirements and guidelines that apply to your bioscience project. This could involve conducting thorough research and consulting with regulatory authorities or industry experts.
03
Create a detailed timeline or project plan for the regulatory process. This should include key milestones, deadlines, and tasks that need to be accomplished.
04
Carefully review and understand the regulatory forms or applications that need to be filled out. Take note of any specific instructions or supporting documents required.
05
Begin filling out the necessary forms or applications, ensuring that all information provided is accurate and complete. Double-check for any errors or missing information before submission.
06
Prepare any supporting documents or data that need to accompany the forms or applications. This may include scientific studies, safety assessments, or clinical trial data.
07
Seek expert guidance or consultation if needed. If you are unsure about any aspect of the regulatory process, it is always beneficial to seek advice from experienced professionals or regulatory consultants.
08
Submit the filled-out forms and applications along with the necessary supporting documents to the appropriate regulatory authorities. Make sure to keep copies of all submitted materials for your records.
09
Monitor the progress of your regulatory submission and follow up with any additional information or clarifications requested by the authorities.
10
Lastly, once your bioscience regulatory process is complete and approved, ensure ongoing compliance with all regulatory requirements by implementing proper monitoring, reporting, and documentation procedures.
Who Needs Planning Your Bioscience Regulatory?
01
Biotechnology companies developing new pharmaceuticals or medical devices.
02
Academic or research institutions involved in bioscience research or development.
03
Contract research organizations (CROs) assisting with clinical trials or regulatory submissions.
04
Regulatory affairs professionals or consultants specializing in bioscience regulations.
05
Government agencies responsible for overseeing bioscience and healthcare regulations.
06
Investors or stakeholders in bioscience projects who need to assess regulatory compliance and potential risks.
07
Healthcare professionals or practitioners seeking regulatory approval for innovative treatment methods or devices.
08
Startups or entrepreneurs looking to enter the bioscience market and navigate regulatory hurdles.
09
Manufacturers or distributors of bioscience products requiring regulatory approval before market launch.
10
Any individual or organization involved in bioscience-related activities that fall under regulatory oversight.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I make changes in planning your bioscience regulatory?
With pdfFiller, it's easy to make changes. Open your planning your bioscience regulatory in the editor, which is very easy to use and understand. When you go there, you'll be able to black out and change text, write and erase, add images, draw lines, arrows, and more. You can also add sticky notes and text boxes.
Can I sign the planning your bioscience regulatory electronically in Chrome?
Yes. You can use pdfFiller to sign documents and use all of the features of the PDF editor in one place if you add this solution to Chrome. In order to use the extension, you can draw or write an electronic signature. You can also upload a picture of your handwritten signature. There is no need to worry about how long it takes to sign your planning your bioscience regulatory.
Can I edit planning your bioscience regulatory on an Android device?
The pdfFiller app for Android allows you to edit PDF files like planning your bioscience regulatory. Mobile document editing, signing, and sending. Install the app to ease document management anywhere.
What is planning your bioscience regulatory?
Planning your bioscience regulatory involves outlining the steps, processes, and strategies to ensure compliance with regulations in the bioscience industry.
Who is required to file planning your bioscience regulatory?
All companies operating in the bioscience industry are required to file planning your bioscience regulatory.
How to fill out planning your bioscience regulatory?
To fill out planning your bioscience regulatory, companies need to provide detailed information about their operations, products, and compliance measures.
What is the purpose of planning your bioscience regulatory?
The purpose of planning your bioscience regulatory is to ensure that companies in the bioscience industry follow regulations to protect public health and the environment.
What information must be reported on planning your bioscience regulatory?
Information such as production processes, waste management plans, product safety measures, and regulatory compliance strategies must be reported on planning your bioscience regulatory.
Fill out your planning your bioscience regulatory online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Planning Your Bioscience Regulatory is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.





















