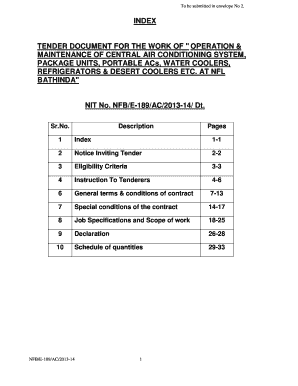
Get the free ETIB Protocol Development Process DRAFT - National Cancer bb - ccrod cancer
Show details
SOP#: ADCR13 Clinical Center Offsite Patient Registration Version #: 1.0 Next Review Date: 10/2017 Approved Date: 10/2015 Review Interval Period: Biennial NCI Clinical Director Signature: William
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign etib protocol development process

Edit your etib protocol development process form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your etib protocol development process form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing etib protocol development process online
To use our professional PDF editor, follow these steps:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit etib protocol development process. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out etib protocol development process

How to fill out etib protocol development process?
01
Start by understanding the purpose and objectives of the etib protocol. This will provide guidance on what information needs to be included and how it should be structured.
02
Identify the key stakeholders involved in the protocol development process. These may include researchers, regulatory bodies, ethics committees, and other relevant parties.
03
Review any existing protocols or guidelines that may be applicable to the specific research or project. This will help ensure that all necessary information is included and that any requirements are met.
04
Gather the relevant data and information needed to complete the protocol. This may involve conducting literature reviews, consulting subject matter experts, and collecting any necessary data or documentation.
05
Structure the protocol in a logical and organized manner, using headings and subheadings to clearly outline each section. Common sections in an etib protocol may include background information, study design, participant eligibility criteria, data collection procedures, and ethical considerations.
06
Write clear and concise descriptions for each section, ensuring that all necessary details are included. Use language that is easily understandable to both experts and non-experts in the field.
07
Regularly review and revise the protocol as needed. This may involve incorporating feedback from stakeholders, addressing any changes in regulations or guidelines, and ensuring that the protocol reflects the most current information and best practices.
Who needs etib protocol development process?
01
Researchers and scientists who are planning to conduct research involving human subjects or animals may need to complete an etib protocol. This protocol helps ensure that the research is conducted in an ethical and responsible manner.
02
Institutions and organizations that oversee research activities may require researchers to submit an etib protocol for review and approval. This can include ethics committees, regulatory bodies, and funding agencies.
03
Participants in the research study or project may benefit from the etib protocol development process as it helps protect their rights and interests. It ensures that their privacy and confidentiality are safeguarded, and that any potential risks or harms are minimized.
04
Society as a whole benefits from the etib protocol development process by promoting ethical research practices and preventing potential harm to individuals and communities. It helps ensure that research is conducted in a responsible and accountable manner.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
Where do I find etib protocol development process?
It's simple using pdfFiller, an online document management tool. Use our huge online form collection (over 25M fillable forms) to quickly discover the etib protocol development process. Open it immediately and start altering it with sophisticated capabilities.
How do I make changes in etib protocol development process?
pdfFiller not only lets you change the content of your files, but you can also change the number and order of pages. Upload your etib protocol development process to the editor and make any changes in a few clicks. The editor lets you black out, type, and erase text in PDFs. You can also add images, sticky notes, and text boxes, as well as many other things.
Can I edit etib protocol development process on an iOS device?
You can. Using the pdfFiller iOS app, you can edit, distribute, and sign etib protocol development process. Install it in seconds at the Apple Store. The app is free, but you must register to buy a subscription or start a free trial.
Fill out your etib protocol development process online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Etib Protocol Development Process is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.