Get the free CRF TRANSMITTAL FORM End of Study Data - Planet 2
Show details
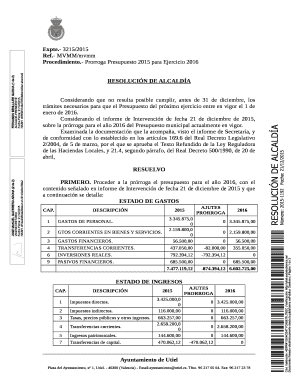
CRF TRANSMITTAL FORM End of Study Data Send completed copy of CRF pages to NHSBTCSU, Long Road, Cambridge, CB2 2PT Or Fax +44 (0)1223 588 136 Or Email: planet2 nest.NHS.UK Clinical Trial Name Planet
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign crf transmittal form end

Edit your crf transmittal form end form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your crf transmittal form end form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing crf transmittal form end online
Use the instructions below to start using our professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit crf transmittal form end. Add and change text, add new objects, move pages, add watermarks and page numbers, and more. Then click Done when you're done editing and go to the Documents tab to merge or split the file. If you want to lock or unlock the file, click the lock or unlock button.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
With pdfFiller, it's always easy to work with documents. Check it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out crf transmittal form end

Point by point, here is how to fill out the CRF Transmittal Form:
01
Start by gathering all the necessary information and documents required for the form, such as the project details, recipient information, and any attachments you need to include.
02
Begin filling out the form by entering the project details, including the project title, identification number, and the name of the organization or agency responsible for the project.
03
Provide the recipient information, which typically includes the name, contact details, and address of the person or organization to whom you are transmitting the CRF.
04
Fill in any additional information requested on the form, such as the date of transmittal, document numbers, or any specific instructions provided.
05
If there are any attachments or supporting documents, make sure to mention them on the form and include them with the transmittal.
06
Double-check all the information you have entered to ensure accuracy and completeness.
07
Sign and date the form, indicating your acceptance of the content being transmitted and your accountability for the information provided.
08
Keep a copy of the completed CRF Transmittal Form for your records before sending the original to the intended recipient.
Who needs the CRF Transmittal Form?
01
The CRF Transmittal Form is typically used in construction and engineering projects where documentation needs to be transmitted from one party to another. It may be required by the architect, general contractor, or project manager.
02
Government agencies, such as those involved in infrastructure development or public works, often require the use of the CRF Transmittal Form for accountability and record-keeping purposes.
03
Any project or organization that follows a formal document control process may use the CRF Transmittal Form to ensure proper tracking and distribution of information.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify crf transmittal form end without leaving Google Drive?
People who need to keep track of documents and fill out forms quickly can connect PDF Filler to their Google Docs account. This means that they can make, edit, and sign documents right from their Google Drive. Make your crf transmittal form end into a fillable form that you can manage and sign from any internet-connected device with this add-on.
How can I edit crf transmittal form end on a smartphone?
You can easily do so with pdfFiller's apps for iOS and Android devices, which can be found at the Apple Store and the Google Play Store, respectively. You can use them to fill out PDFs. We have a website where you can get the app, but you can also get it there. When you install the app, log in, and start editing crf transmittal form end, you can start right away.
How do I complete crf transmittal form end on an iOS device?
Download and install the pdfFiller iOS app. Then, launch the app and log in or create an account to have access to all of the editing tools of the solution. Upload your crf transmittal form end from your device or cloud storage to open it, or input the document URL. After filling out all of the essential areas in the document and eSigning it (if necessary), you may save it or share it with others.
What is CRF transmittal form end?
The CRF transmittal form end is a document used in clinical research to summarize and transmit case report forms (CRFs) to regulatory bodies or sponsors.
Who is required to file CRF transmittal form end?
Researchers, clinical trial sites, or sponsors who are conducting clinical trials that require submission of case report forms are typically required to file the CRF transmittal form end.
How to fill out CRF transmittal form end?
To fill out the CRF transmittal form end, you should enter the study title, protocol number, the details of the site, contact information, the list of CRFs being transmitted, and any relevant comments or notes required by the regulatory authority.
What is the purpose of CRF transmittal form end?
The purpose of the CRF transmittal form end is to organize and ensure accurate submission of case report forms for review and data management, facilitating compliance with regulatory requirements.
What information must be reported on CRF transmittal form end?
Information that must be reported includes the study title, protocol number, site identifier, names of the principal investigator and site staff, list of CRFs submitted, dates of submission, and any applicable comments.
Fill out your crf transmittal form end online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Crf Transmittal Form End is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.