Get the free Enthalpy of Hydration and Solution
Show details
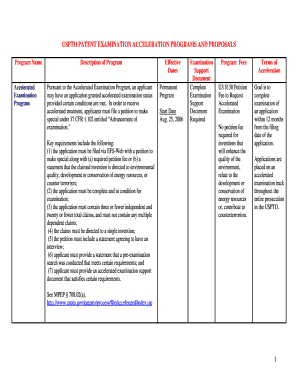
Enthalpy of Hydration and Solution INSTRUCTOR RESOURCES By Dale A. Hammond, PhD, Brigham Young University Hawaii LEARNING OBJECTIVES: Qualitatively correlate the Sold of several compounds with their
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign enthalpy of hydration and

Edit your enthalpy of hydration and form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your enthalpy of hydration and form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit enthalpy of hydration and online
To use the professional PDF editor, follow these steps below:
1
Sign into your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit enthalpy of hydration and. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Register for an account and see for yourself!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out enthalpy of hydration and

How to Fill Out Enthalpy of Hydration:
01
Start by gathering all the necessary data related to the substance you are studying. This includes the molar mass of the compound, the initial and final temperatures of the solution, and the amount of water used.
02
Calculate the change in temperature (∆T) by subtracting the initial temperature from the final temperature.
03
Determine the number of moles of water used in the reaction. This can be calculated by dividing the mass of water used by the molar mass of water (18 g/mol).
04
Calculate the enthalpy change (∆H) using the formula ∆H = q/moles of water, where q is the heat absorbed or released during the reaction. Ensure that the units of all the values used are consistent.
05
Finally, record the value of enthalpy of hydration as a positive or negative value, depending on whether the reaction is exothermic (negative ∆H) or endothermic (positive ∆H).
Who Needs Enthalpy of Hydration:
01
Chemistry Students: Enthalpy of hydration is an important concept in chemistry, especially in the study of aqueous solutions and the behavior of solutes. It helps students understand the thermodynamics of hydration processes and how they contribute to overall enthalpy changes.
02
Researchers: Scientists conducting experiments or investigations related to thermodynamics, solution chemistry, or specific compounds often require enthalpy of hydration data. This information helps them determine the stability, reactivity, and energy changes associated with hydration reactions.
03
Engineers: In industries where understanding the behavior of solutes in solution is crucial, such as pharmaceuticals or chemical manufacturing, engineers might rely on enthalpy of hydration measurements. This data aids in designing efficient production processes and optimizing reaction conditions.
04
Environmental Scientists: Enthalpy of hydration is essential for understanding the solubility and transport of chemicals in the environment, such as pollutants. Environmental scientists might use this information to assess the potential impact of substances on ecosystems or to model their fate in aquatic systems.
Overall, anyone studying or working in the fields of chemistry, research, engineering, or environmental science may need to consider enthalpy of hydration to gain insights into the energetics and behavior of substances in solution.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify enthalpy of hydration and without leaving Google Drive?
pdfFiller and Google Docs can be used together to make your documents easier to work with and to make fillable forms right in your Google Drive. The integration will let you make, change, and sign documents, like enthalpy of hydration and, without leaving Google Drive. Add pdfFiller's features to Google Drive, and you'll be able to do more with your paperwork on any internet-connected device.
How do I edit enthalpy of hydration and online?
pdfFiller allows you to edit not only the content of your files, but also the quantity and sequence of the pages. Upload your enthalpy of hydration and to the editor and make adjustments in a matter of seconds. Text in PDFs may be blacked out, typed in, and erased using the editor. You may also include photos, sticky notes, and text boxes, among other things.
How do I fill out the enthalpy of hydration and form on my smartphone?
The pdfFiller mobile app makes it simple to design and fill out legal paperwork. Complete and sign enthalpy of hydration and and other papers using the app. Visit pdfFiller's website to learn more about the PDF editor's features.
What is enthalpy of hydration and?
Enthalpy of hydration is the amount of heat released or absorbed when one mole of a substance dissolves in water.
Who is required to file enthalpy of hydration and?
Enthalpy of hydration is typically studied and calculated by chemists and researchers.
How to fill out enthalpy of hydration and?
Enthalpy of hydration can be filled out by conducting experiments to measure the heat change during dissolution of a substance in water.
What is the purpose of enthalpy of hydration and?
The purpose of enthalpy of hydration is to understand the energetics of the dissolution process and the interactions between solute and solvent molecules.
What information must be reported on enthalpy of hydration and?
The information reported on enthalpy of hydration includes the substance being dissolved, the amount of heat released or absorbed, and the conditions of the experiment.
Fill out your enthalpy of hydration and online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Enthalpy Of Hydration And is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.