Get the free Gases Boyle's Law
Show details
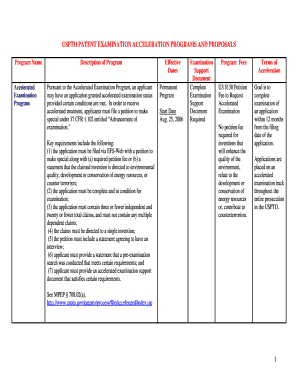
Gases: Boyle's Law INSTRUCTOR RESOURCES The CCI Initiative Learning Objectives ! ! Introduce the concepts and units of pressure, volume and temperature. Experimentally determine the relationship between
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign gases boyles law

Edit your gases boyles law form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your gases boyles law form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit gases boyles law online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit gases boyles law. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
With pdfFiller, it's always easy to work with documents. Try it out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out gases boyles law

How to fill out gases Boyle's Law:
01
Understand the concept: Before filling out gases Boyle's Law, it is essential to comprehend the underlying principle. Boyle's Law states that the pressure and volume of a gas are inversely proportional to each other at a constant temperature. This means that as the volume of a gas increases, the pressure decreases, and vice versa.
02
Gather necessary information: To fill out gases Boyle's Law, you will need specific information about the gas and its initial and final conditions. This includes the initial volume, initial pressure, final volume, and final pressure of the gas.
03
Apply the formula: Boyle's Law can be mathematically represented as P₁V₁ = P₂V₂, where P₁ and P₂ are the initial and final pressures, and V₁ and V₂ are the initial and final volumes, respectively.
04
Identify and substitute the values: Take the given values for the initial and final conditions of the gas and substitute them into the Boyle's Law equation. Ensure that the units of pressure and volume are consistent (e.g., both in atm or both in kPa) for accurate calculations.
05
Solve for the unknown: By rearranging the Boyle's Law equation, you can find the missing value if given the other three. For example, if you need to find the final pressure (P₂), multiply the initial pressure (P₁) by the initial volume (V₁) and divide the result by the final volume (V₂).
06
Perform the calculations: Use a calculator or manually compute the values to obtain the answer. Double-check your calculations for accuracy.
Who needs gases Boyle's Law:
01
Students studying chemistry or physics: Boyle's Law is a fundamental concept in both chemistry and physics. Students pursuing these subjects will need to understand and apply Boyle's Law for various calculations and experiments involving gases.
02
Scientists and researchers: In scientific fields, gases are frequently encountered and controlled. Scientists and researchers use Boyle's Law to study the behavior of gases under different conditions, analyze their properties, and make accurate predictions and conclusions.
03
Engineers and technicians: Understanding Boyle's Law is valuable for engineers and technicians who deal with gas systems, such as HVAC technicians, chemical engineers, or industrial process engineers. They rely on Boyle's Law to design and optimize gas systems, determine appropriate component sizes, and troubleshoot any issues that arise.
Overall, anyone working with gases or studying the properties of gases will find Boyle's Law to be a crucial concept in their respective fields.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify gases boyles law without leaving Google Drive?
By integrating pdfFiller with Google Docs, you can streamline your document workflows and produce fillable forms that can be stored directly in Google Drive. Using the connection, you will be able to create, change, and eSign documents, including gases boyles law, all without having to leave Google Drive. Add pdfFiller's features to Google Drive and you'll be able to handle your documents more effectively from any device with an internet connection.
How do I edit gases boyles law online?
With pdfFiller, you may not only alter the content but also rearrange the pages. Upload your gases boyles law and modify it with a few clicks. The editor lets you add photos, sticky notes, text boxes, and more to PDFs.
Can I edit gases boyles law on an iOS device?
Yes, you can. With the pdfFiller mobile app, you can instantly edit, share, and sign gases boyles law on your iOS device. Get it at the Apple Store and install it in seconds. The application is free, but you will have to create an account to purchase a subscription or activate a free trial.
What is gases Boyles law?
Boyle's law states that the pressure of a gas is inversely proportional to its volume at a constant temperature.
Who is required to file gases Boyles law?
Any individual or entity handling gases is required to comply with Boyle's law.
How to fill out gases Boyles law?
To fill out Boyle's law, one needs to measure the pressure and volume of a gas at a constant temperature and calculate the corresponding values.
What is the purpose of gases Boyles law?
The purpose of Boyle's law is to describe the relationship between the pressure and volume of a gas under constant temperature.
What information must be reported on gases Boyles law?
The information to be reported on Boyle's law includes the pressure, volume, and temperature of the gas, as well as the calculation of the constant.
Fill out your gases boyles law online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Gases Boyles Law is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.