Get the free Pharmacovigilance Regulations - diaglobal
Show details
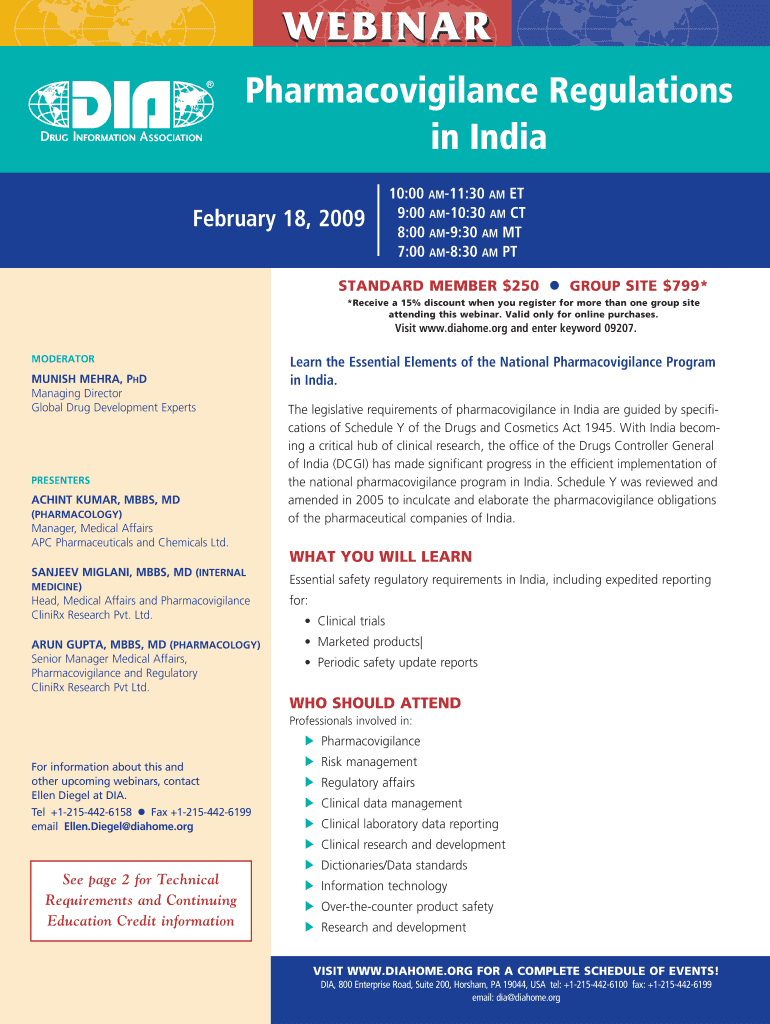
WEBINAR Pharmacovigilance Regulations in India February 18, 2009 10:00 AM11:30 AM ET 9:00 AM10:30 AM CT 8:00 AM9:30 AM MT 7:00 AM8:30 AM PT STANDARD MEMBER $250 GROUP SITE $799* *Receive a 15% discount
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign pharmacovigilance regulations - diaglobal

Edit your pharmacovigilance regulations - diaglobal form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your pharmacovigilance regulations - diaglobal form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit pharmacovigilance regulations - diaglobal online
Follow the steps below to take advantage of the professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit pharmacovigilance regulations - diaglobal. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Select it in the list of your records. Then, move the cursor to the right toolbar and choose one of the available exporting methods: save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud.
With pdfFiller, dealing with documents is always straightforward. Now is the time to try it!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out pharmacovigilance regulations - diaglobal

How to fill out pharmacovigilance regulations:
01
Understand the purpose: Familiarize yourself with the objectives of pharmacovigilance regulations, which are aimed at ensuring the safety and monitoring of pharmaceutical products throughout their lifecycle.
02
Study the applicable regulations: Research and review the specific pharmacovigilance regulations that are relevant to your region or country. This may include guidelines provided by regulatory authorities such as the FDA (Food and Drug Administration) in the United States or the EMA (European Medicines Agency) in Europe.
03
Determine your responsibilities: Identify your role within the pharmacovigilance framework. This could be as a drug manufacturer, marketing authorization holder, healthcare professional, or a member of the pharmacovigilance team.
04
Establish a pharmacovigilance system: Develop a comprehensive system to collect, evaluate, and report adverse drug reactions (ADRs) associated with the use of your pharmaceutical products. This may involve setting up processes for ADR reporting, signal detection, risk management, and other activities required by the regulations.
05
Train staff: Ensure that your team members are trained and knowledgeable about pharmacovigilance regulations. They should understand their specific roles and responsibilities in terms of reporting and monitoring adverse events.
06
Implement effective documentation: Maintain accurate and up-to-date records of pharmacovigilance activities, including ADR reports, safety signals, risk management plans, and any interactions with regulatory authorities. This documentation is essential for compliance and may be subject to inspections or audits.
07
Engage in continuous monitoring and improvement: Regularly review your pharmacovigilance processes to identify any areas for improvement. Stay updated with relevant regulatory changes and adapt your practices accordingly.
Who needs pharmacovigilance regulations:
01
Pharmaceutical companies: Drug manufacturers are required to comply with pharmacovigilance regulations to ensure the safety of their products. They must establish systems for post-marketing surveillance, reporting adverse events, and implementing risk management plans.
02
Regulatory authorities: Government agencies responsible for drug regulation, such as the FDA or EMA, have a crucial role in enforcing pharmacovigilance regulations. They set guidelines and standards, review safety data, and oversee compliance by pharmaceutical companies.
03
Healthcare professionals: Doctors, nurses, and other healthcare professionals play a role in pharmacovigilance by reporting adverse events they observe or that are reported to them. They contribute to the overall safety monitoring of drugs and help identify potential safety concerns.
04
Patients: Patients, as end-users of pharmaceutical products, are also impacted by pharmacovigilance regulations. They can play an active role by reporting any suspected adverse reactions to their healthcare providers or directly to pharmacovigilance authorities.
05
Marketing Authorization Holders (MAHs): These are individuals or organizations that hold the legal responsibility for authorizing the marketing of a pharmaceutical product. MAHs must comply with pharmacovigilance regulations to ensure the ongoing safety surveillance of their products.
In conclusion, filling out pharmacovigilance regulations involves understanding the purpose, studying applicable regulations, determining responsibilities, establishing a pharmacovigilance system, training staff, implementing effective documentation, and engaging in continuous monitoring and improvement. The key stakeholders for pharmacovigilance regulations include pharmaceutical companies, regulatory authorities, healthcare professionals, patients, and Marketing Authorization Holders.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I modify pharmacovigilance regulations - diaglobal without leaving Google Drive?
Simplify your document workflows and create fillable forms right in Google Drive by integrating pdfFiller with Google Docs. The integration will allow you to create, modify, and eSign documents, including pharmacovigilance regulations - diaglobal, without leaving Google Drive. Add pdfFiller’s functionalities to Google Drive and manage your paperwork more efficiently on any internet-connected device.
How do I complete pharmacovigilance regulations - diaglobal online?
pdfFiller has made it easy to fill out and sign pharmacovigilance regulations - diaglobal. You can use the solution to change and move PDF content, add fields that can be filled in, and sign the document electronically. Start a free trial of pdfFiller, the best tool for editing and filling in documents.
How can I fill out pharmacovigilance regulations - diaglobal on an iOS device?
Download and install the pdfFiller iOS app. Then, launch the app and log in or create an account to have access to all of the editing tools of the solution. Upload your pharmacovigilance regulations - diaglobal from your device or cloud storage to open it, or input the document URL. After filling out all of the essential areas in the document and eSigning it (if necessary), you may save it or share it with others.
What is pharmacovigilance regulations?
Pharmacovigilance regulations are rules and guidelines put in place to monitor and evaluate the safety of pharmaceutical drugs and medical devices.
Who is required to file pharmacovigilance regulations?
Pharmaceutical companies and medical device manufacturers are required to file pharmacovigilance regulations.
How to fill out pharmacovigilance regulations?
Pharmacovigilance regulations can be filled out by submitting adverse event reports, periodic safety update reports, and risk management plans.
What is the purpose of pharmacovigilance regulations?
The purpose of pharmacovigilance regulations is to ensure the safety of patients by monitoring and reporting adverse effects of pharmaceutical drugs and medical devices.
What information must be reported on pharmacovigilance regulations?
Information such as adverse events, patient outcomes, and drug interactions must be reported on pharmacovigilance regulations.
Fill out your pharmacovigilance regulations - diaglobal online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Pharmacovigilance Regulations - Diaglobal is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















