Get the free agilent iq oq
Show details
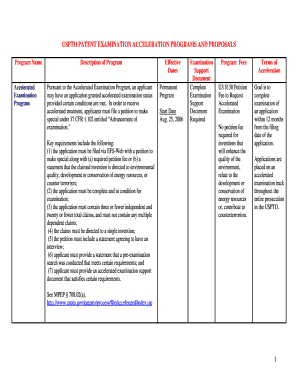
Equipment Qualification Plan (ESP) GC Agent 7890/7820 Agent 6890/6850/5890 ESP : Agent Recommended EQ PGC (CTC) Agent Recommended EQ PGC : 2012 (ESP) OF IQ 3 (DQ) (RQ) (IQ) (OF) IQ OF (ACE) OF ESP
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign agilent iq oq

Edit your agilent iq oq form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your agilent iq oq form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing agilent iq oq online
Use the instructions below to start using our professional PDF editor:
1
Log in to account. Click Start Free Trial and register a profile if you don't have one yet.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit agilent iq oq. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
With pdfFiller, dealing with documents is always straightforward.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out agilent iq oq

01
The first step to fill out the Agilent IQ OQ is to gather all the necessary information and documents. This includes the Agilent IQ OQ template, any equipment manuals, and any relevant calibration data.
02
Next, review the Agilent IQ OQ template and familiarize yourself with the different sections and requirements. It is important to understand the purpose and scope of the IQ OQ.
03
Fill in the basic information section of the Agilent IQ OQ, including details such as the equipment model, serial number, and location. Also, include the names and roles of the individuals responsible for conducting the IQ OQ.
04
Proceed to the installation qualification (IQ) section. This involves verifying and documenting that the equipment has been installed correctly and according to the manufacturer's instructions. Follow the instructions on the template to document the necessary installation checks.
05
Move on to the operational qualification (OQ) section. This involves testing and verifying that the equipment functions properly and meets the specified performance criteria. Again, follow the instructions on the template to document the required tests and results.
06
Ensure that you include any necessary calibration data in the IQ OQ document. This may include calibrating measurement equipment used during the qualification process or noting the latest calibration dates for the equipment being qualified.
07
Review and double-check all the information filled out in the Agilent IQ OQ to ensure accuracy and completeness. Make sure that all sections have been properly addressed and documented.
08
Once the Agilent IQ OQ is filled out, it may need to be reviewed and approved by relevant parties, such as quality assurance personnel or equipment owners.
09
Save the completed Agilent IQ OQ document in a secure and accessible location for future reference, such as in a document management system or a designated folder.
10
Finally, communicate any findings or issues identified during the IQ OQ process to the appropriate personnel, and take necessary actions to address them.
Answer to "Who needs Agilent IQ OQ?":
The Agilent IQ OQ is typically required by organizations or laboratories that use Agilent equipment and need to ensure that it is properly installed, qualified, and meets the manufacturer's specifications. This may include pharmaceutical companies, research institutions, manufacturing facilities, or any organization that relies on accurate and reliable analytical instrumentation. The Agilent IQ OQ helps to validate the performance and compliance of the equipment, ensuring confidence in its accuracy and reliability.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I send agilent iq oq for eSignature?
Once your agilent iq oq is ready, you can securely share it with recipients and collect eSignatures in a few clicks with pdfFiller. You can send a PDF by email, text message, fax, USPS mail, or notarize it online - right from your account. Create an account now and try it yourself.
Can I sign the agilent iq oq electronically in Chrome?
Yes, you can. With pdfFiller, you not only get a feature-rich PDF editor and fillable form builder but a powerful e-signature solution that you can add directly to your Chrome browser. Using our extension, you can create your legally-binding eSignature by typing, drawing, or capturing a photo of your signature using your webcam. Choose whichever method you prefer and eSign your agilent iq oq in minutes.
How do I complete agilent iq oq on an iOS device?
Make sure you get and install the pdfFiller iOS app. Next, open the app and log in or set up an account to use all of the solution's editing tools. If you want to open your agilent iq oq, you can upload it from your device or cloud storage, or you can type the document's URL into the box on the right. After you fill in all of the required fields in the document and eSign it, if that is required, you can save or share it with other people.
What is agilent iq oq?
Agilent IQ OQ stands for Installation Qualification and Operation Qualification. It is a set of documented tests that ensure a piece of equipment, such as an Agilent instrument, is properly installed and functions correctly.
Who is required to file agilent iq oq?
Any organization using Agilent instruments in a regulated industry, such as pharmaceuticals or biotechnology, is required to perform and document IQ OQ testing.
How to fill out agilent iq oq?
To fill out Agilent IQ OQ, follow the testing protocols provided by Agilent for installation qualification and operation qualification. Document the results of each test performed.
What is the purpose of agilent iq oq?
The purpose of Agilent IQ OQ is to ensure that Agilent instruments are installed correctly and operating as intended. This helps to guarantee the accuracy and reliability of data generated by the instruments.
What information must be reported on agilent iq oq?
Agilent IQ OQ reports must include details of the tests performed, the results of the tests, any deviations from expected outcomes, and any corrective actions taken to address deviations.
Fill out your agilent iq oq online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Agilent Iq Oq is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.