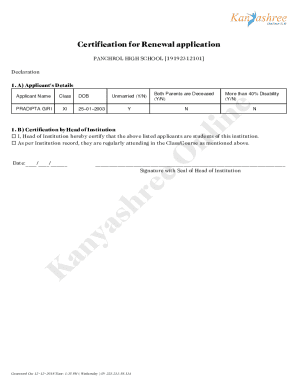
Get the free Clinical Trial Supply Management Webinar
Show details
This document provides an overview of the Clinical Trial Supply Management processes and solutions offered by SAP for the life sciences industry, detailing the challenges, solutions, and benefits.
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign clinical trial supply management

Edit your clinical trial supply management form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your clinical trial supply management form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit clinical trial supply management online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Prepare a file. Use the Add New button to start a new project. Then, using your device, upload your file to the system by importing it from internal mail, the cloud, or adding its URL.
3
Edit clinical trial supply management. Text may be added and replaced, new objects can be included, pages can be rearranged, watermarks and page numbers can be added, and so on. When you're done editing, click Done and then go to the Documents tab to combine, divide, lock, or unlock the file.
4
Save your file. Choose it from the list of records. Then, shift the pointer to the right toolbar and select one of the several exporting methods: save it in multiple formats, download it as a PDF, email it, or save it to the cloud.
pdfFiller makes dealing with documents a breeze. Create an account to find out!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out clinical trial supply management

How to fill out Clinical Trial Supply Management Webinar
01
Visit the official website of the Clinical Trial Supply Management Webinar.
02
Locate the registration section for the webinar.
03
Fill out the required personal information including name, email, and organization.
04
Select the preferred date and time for attending the webinar.
05
Review and agree to any terms and conditions provided.
06
Submit the registration form.
07
Check your email for a confirmation message with a link to the webinar.
Who needs Clinical Trial Supply Management Webinar?
01
Clinical trial managers
02
Supply chain professionals
03
Pharmaceutical researchers
04
Regulatory affairs specialists
05
Clinical operations teams
06
Healthcare professionals involved in trials
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Clinical Trial Supply Management Webinar?
The Clinical Trial Supply Management Webinar is an educational online seminar that provides insights and best practices for managing the supply of materials and medications used in clinical trials.
Who is required to file Clinical Trial Supply Management Webinar?
Clinical trial sponsors, researchers, and supply chain managers involved in the planning, execution, and oversight of clinical trials are typically required to file for the Clinical Trial Supply Management Webinar.
How to fill out Clinical Trial Supply Management Webinar?
To fill out the Clinical Trial Supply Management Webinar, participants should complete the registration form with their relevant details, including name, organization, and any required background information related to their role in clinical trials.
What is the purpose of Clinical Trial Supply Management Webinar?
The purpose of the Clinical Trial Supply Management Webinar is to educate participants on effective strategies for managing clinical trial supplies, ensuring compliance, and improving the efficiency of trial operations.
What information must be reported on Clinical Trial Supply Management Webinar?
Participants must report on their roles in clinical trials, relevant experiences, supply chain challenges faced, and any specific areas of interest or questions they hope to address during the webinar.
Fill out your clinical trial supply management online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Clinical Trial Supply Management is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.