Get the free CHM111 Lab Enthalpy of Hydration of Sodium Acetate - blogs nvcc
Show details
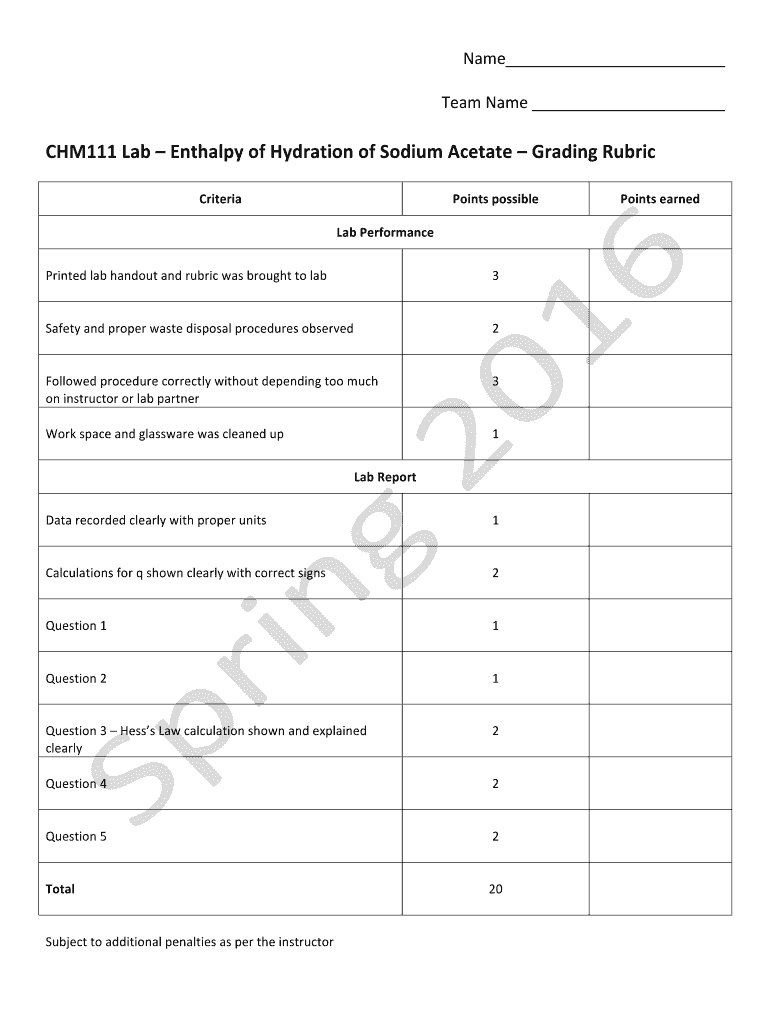
Name Team Name CHM111 Lab Enthalpy of Hydration of Sodium Acetate Grading Rubric Criteria Points possible Points earned Lab Performance Printed lab handout and rubric was brought to lab Safety and
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign chm111 lab enthalpy of

Edit your chm111 lab enthalpy of form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your chm111 lab enthalpy of form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing chm111 lab enthalpy of online
To use our professional PDF editor, follow these steps:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit chm111 lab enthalpy of. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. When you find your file in the docs list, click on its name and choose how you want to save it. To get the PDF, you can save it, send an email with it, or move it to the cloud.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out chm111 lab enthalpy of

How to fill out chm111 lab enthalpy of?
01
Read the lab instructions carefully: It is essential to understand the purpose of the lab and the specific steps involved. Make sure to follow any safety precautions mentioned.
02
Gather the necessary equipment: Check the lab instructions for a list of materials and equipment needed. Collect all the items before starting the experiment to ensure a smooth workflow.
03
Set up the experiment: Follow the given instructions to set up the apparatus or create the required conditions. This may involve measuring and mixing specific chemicals, adjusting temperature or pressure, or assembling apparatus.
04
Carry out the experiment: Follow the outlined procedure step by step. Record observations, measurements, and data accurately. Take note of any changes, color, formation of precipitate, or any other relevant information.
05
Calculate the enthalpy change: Once the experiment is complete, retrieve the recorded data and use it to determine the change in enthalpy. This may involve applying appropriate formulas, equations, or using graphical methods.
06
Analyze the results: Interpret the calculated enthalpy change and draw conclusions based on your findings. Consider any sources of error and their potential impact on the results. Discuss possible improvements or future research directions.
Who needs chm111 lab enthalpy of?
01
Chemistry students: Chm111 lab enthalpy of is specifically designed for students studying chemistry. It provides them with practical experience in measuring and calculating enthalpy changes, deepening their understanding of thermodynamics and chemical reactions.
02
Scientists and researchers: While chm111 lab enthalpy of is primarily aimed at educational purposes, researchers and scientists working in fields such as chemical engineering, environmental science, or materials science may also need to measure enthalpy changes in their experiments.
03
Professionals in related industries: Individuals working in industries that involve chemical processes, such as pharmaceuticals, energy production, or food sciences, may require knowledge of enthalpy changes. Understanding thermodynamics and how to measure enthalpy can be beneficial in optimizing processes and ensuring product quality.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit chm111 lab enthalpy of from Google Drive?
You can quickly improve your document management and form preparation by integrating pdfFiller with Google Docs so that you can create, edit and sign documents directly from your Google Drive. The add-on enables you to transform your chm111 lab enthalpy of into a dynamic fillable form that you can manage and eSign from any internet-connected device.
How do I edit chm111 lab enthalpy of online?
The editing procedure is simple with pdfFiller. Open your chm111 lab enthalpy of in the editor, which is quite user-friendly. You may use it to blackout, redact, write, and erase text, add photos, draw arrows and lines, set sticky notes and text boxes, and much more.
How do I fill out chm111 lab enthalpy of using my mobile device?
On your mobile device, use the pdfFiller mobile app to complete and sign chm111 lab enthalpy of. Visit our website (https://edit-pdf-ios-android.pdffiller.com/) to discover more about our mobile applications, the features you'll have access to, and how to get started.
What is chm111 lab enthalpy of?
Chm111 lab enthalpy is the measurement of heat released or absorbed during a chemical reaction.
Who is required to file chm111 lab enthalpy of?
Students enrolled in CHM111 lab courses are required to fill out chm111 lab enthalpy of.
How to fill out chm111 lab enthalpy of?
To fill out chm111 lab enthalpy, students need to conduct experiments, collect data, and calculate the enthalpy change of the reactions.
What is the purpose of chm111 lab enthalpy of?
The purpose of chm111 lab enthalpy is to help students understand and calculate the energy changes in chemical reactions.
What information must be reported on chm111 lab enthalpy of?
Students must report the reactants, products, initial and final temperatures, mass of reactants, and the calculated enthalpy change.
Fill out your chm111 lab enthalpy of online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

chm111 Lab Enthalpy Of is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.



















