Get the free HIPAA Research Policy-- Research 301
Show details
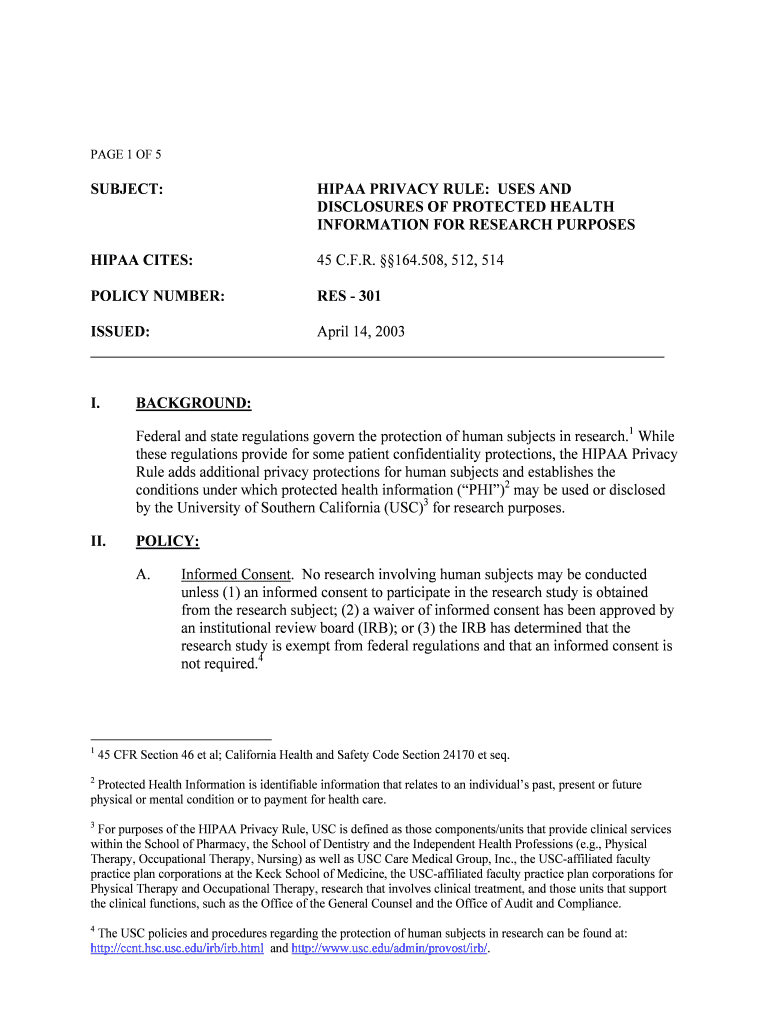
PAGE 1 OF 5 SUBJECT: HIPAA PRIVACY RULE: USES AND DISCLOSURES OF PROTECTED HEALTH INFORMATION FOR RESEARCH PURPOSES HIPAA CITES: 45 C.F.R. 164.508, 512, 514 POLICY NUMBER: RES 301 ISSUED: April 14,
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign hipaa research policy-- research

Edit your hipaa research policy-- research form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your hipaa research policy-- research form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit hipaa research policy-- research online
To use our professional PDF editor, follow these steps:
1
Set up an account. If you are a new user, click Start Free Trial and establish a profile.
2
Simply add a document. Select Add New from your Dashboard and import a file into the system by uploading it from your device or importing it via the cloud, online, or internal mail. Then click Begin editing.
3
Edit hipaa research policy-- research. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
pdfFiller makes working with documents easier than you could ever imagine. Try it for yourself by creating an account!
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out hipaa research policy-- research

How to fill out HIPAA research policy-- research:
01
Familiarize yourself with the HIPAA regulations and guidelines regarding research. This includes understanding the requirements for protecting the privacy and confidentiality of individuals' health information involved in the research.
02
Identify the specific research activities that will involve the use or disclosure of protected health information (PHI). Be clear about the purpose of the research and how the PHI will be used to fulfill that purpose.
03
Develop a comprehensive and detailed HIPAA research policy that outlines the procedures and safeguards you will implement to ensure compliance with HIPAA regulations. Include provisions for obtaining authorization from individuals for the use of their PHI in the research, as well as procedures for securely storing and transmitting the PHI.
04
Train all individuals involved in the research on the HIPAA research policy and their responsibilities in protecting PHI. This includes researchers, staff members, and any other individuals who may come into contact with PHI during the research process.
05
Implement the HIPAA research policy and regularly monitor and assess its effectiveness. Conduct periodic audits to ensure compliance and address any identified issues or areas for improvement.
06
Continuously stay updated on any changes or updates to the HIPAA regulations and adjust your research policy accordingly to remain in compliance.
Who needs HIPAA research policy-- research:
01
Any individuals or organizations conducting research that involves the use or disclosure of protected health information (PHI) are required to have a HIPAA research policy in place. This includes academic institutions, medical centers, research institutes, pharmaceutical companies, and individual researchers.
02
Research involving PHI could include clinical trials, retrospective studies, surveys, and any other research activities that require access to individuals' health information.
03
Entities or individuals who are directly or indirectly involved in the research process, such as research coordinators, data managers, principal investigators, and institutional review boards, also need to be knowledgeable and comply with the HIPAA research policy.
04
The HIPAA research policy ensures that appropriate measures are in place to safeguard the privacy and confidentiality of individuals' health information, as mandated by HIPAA regulations. It helps to prevent unauthorized access, use, or disclosure of PHI during the research process.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How can I edit hipaa research policy-- research from Google Drive?
You can quickly improve your document management and form preparation by integrating pdfFiller with Google Docs so that you can create, edit and sign documents directly from your Google Drive. The add-on enables you to transform your hipaa research policy-- research into a dynamic fillable form that you can manage and eSign from any internet-connected device.
How do I execute hipaa research policy-- research online?
Completing and signing hipaa research policy-- research online is easy with pdfFiller. It enables you to edit original PDF content, highlight, blackout, erase and type text anywhere on a page, legally eSign your form, and much more. Create your free account and manage professional documents on the web.
How do I edit hipaa research policy-- research online?
With pdfFiller, it's easy to make changes. Open your hipaa research policy-- research in the editor, which is very easy to use and understand. When you go there, you'll be able to black out and change text, write and erase, add images, draw lines, arrows, and more. You can also add sticky notes and text boxes.
What is hipaa research policy-- research?
HIPAA research policy is a set of guidelines and regulations related to protecting the privacy and security of health information used in research projects.
Who is required to file hipaa research policy-- research?
Any entity or individual conducting research involving protected health information (PHI) is required to file a HIPAA research policy.
How to fill out hipaa research policy-- research?
HIPAA research policy can be filled out by following the specific guidelines provided by the HIPAA regulations and ensuring that all necessary information is included.
What is the purpose of hipaa research policy-- research?
The purpose of HIPAA research policy is to ensure that the privacy and security of health information used in research projects are protected.
What information must be reported on hipaa research policy-- research?
The HIPAA research policy must include details on how protected health information will be used, stored, and shared during the research project.
Fill out your hipaa research policy-- research online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Hipaa Research Policy-- Research is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















