Get the free E2 Continuous Research Enrollment - College of Charleston - gradschool cofc
Show details
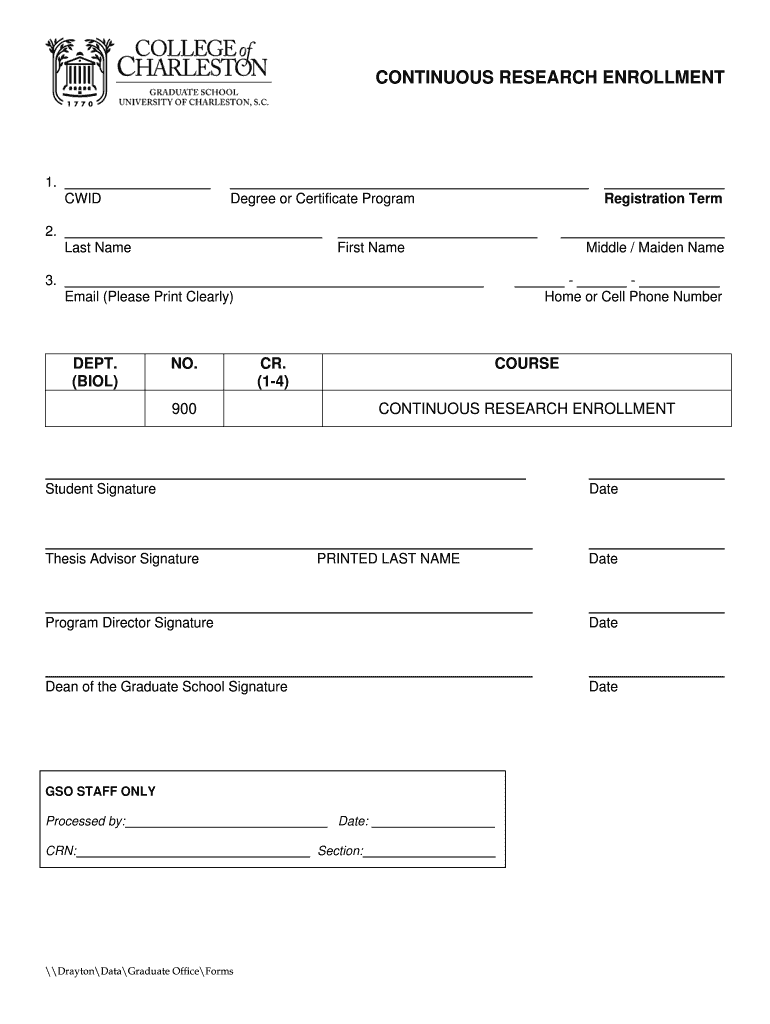
DraytonDataGraduate Officers CONTINUOUS RESEARCH ENROLLMENT 1. CID ...
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign e2 continuous research enrollment

Edit your e2 continuous research enrollment form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your e2 continuous research enrollment form via URL. You can also download, print, or export forms to your preferred cloud storage service.
Editing e2 continuous research enrollment online
Follow the guidelines below to use a professional PDF editor:
1
Register the account. Begin by clicking Start Free Trial and create a profile if you are a new user.
2
Upload a file. Select Add New on your Dashboard and upload a file from your device or import it from the cloud, online, or internal mail. Then click Edit.
3
Edit e2 continuous research enrollment. Rearrange and rotate pages, add new and changed texts, add new objects, and use other useful tools. When you're done, click Done. You can use the Documents tab to merge, split, lock, or unlock your files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out e2 continuous research enrollment

How to fill out e2 continuous research enrollment:
01
Start by obtaining the necessary forms from the research institution or organization overseeing the study. These forms may be available online or can be obtained directly from the research coordinator.
02
Carefully read through the instructions provided with the enrollment forms to ensure you understand the requirements and expectations for participation in the research study.
03
Fill out the personal information section of the enrollment form, including your name, contact details, date of birth, and any other required information. Make sure to double-check the accuracy of the provided information.
04
Provide information about your medical history, including any past or current medical conditions, medications you are taking, and any allergies or sensitivities that may be relevant to the research study.
05
Complete the sections related to your demographic information, such as gender, ethnicity, and race. This information helps researchers ensure a diverse and representative sample.
06
Answer any questions related to your eligibility for the research study. This may include questions about your current health status, lifestyle habits, or previous participation in similar studies.
07
If required, obtain any necessary signatures or consents from legal guardians or authorized representatives. This is especially important for participants who are minors or individuals who may have limited decision-making capacity.
08
Review the completed enrollment form for accuracy and completeness. Make sure that all sections have been filled out properly and that there are no errors or missing information.
09
Submit the filled-out enrollment form as instructed by the research institution. This may involve mailing it back, submitting it electronically, or delivering it in person.
10
Keep a copy of the filled-out enrollment form for your records. This can be helpful for future reference or if any questions arise regarding your participation in the research study.
Who needs e2 continuous research enrollment?
01
Researchers conducting clinical trials or studies that require the enrollment of human participants.
02
Individuals who meet the eligibility criteria and are interested in participating in research studies or clinical trials.
03
Participants who are willing to commit to the requirements and time commitment involved in the research study or clinical trial.
04
Individuals who have provided informed consent and have a clear understanding of the purpose, risks, and benefits of the research study or clinical trial.
05
Participants who are willing to follow the instructions and protocols provided by the research institution and comply with the required procedures and assessments.
06
Individuals with specific medical conditions or demographics that are important for the research study or clinical trial, as determined by the researchers.
07
Participants who are keen on contributing to scientific knowledge and advancements in their respective fields of study.
08
Individuals who are comfortable with sharing their personal and medical information, as required for the research study or clinical trial.
09
Participants who understand that their involvement in the research study or clinical trial is voluntary and can withdraw their consent at any time.
10
Individuals who are willing to communicate openly with the research team, providing any necessary updates or reporting any adverse events experienced during the study.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my e2 continuous research enrollment in Gmail?
pdfFiller’s add-on for Gmail enables you to create, edit, fill out and eSign your e2 continuous research enrollment and any other documents you receive right in your inbox. Visit Google Workspace Marketplace and install pdfFiller for Gmail. Get rid of time-consuming steps and manage your documents and eSignatures effortlessly.
How can I modify e2 continuous research enrollment without leaving Google Drive?
It is possible to significantly enhance your document management and form preparation by combining pdfFiller with Google Docs. This will allow you to generate papers, amend them, and sign them straight from your Google Drive. Use the add-on to convert your e2 continuous research enrollment into a dynamic fillable form that can be managed and signed using any internet-connected device.
How do I execute e2 continuous research enrollment online?
Completing and signing e2 continuous research enrollment online is easy with pdfFiller. It enables you to edit original PDF content, highlight, blackout, erase and type text anywhere on a page, legally eSign your form, and much more. Create your free account and manage professional documents on the web.
What is e2 continuous research enrollment?
E2 continuous research enrollment is a process of continuously updating research data.
Who is required to file e2 continuous research enrollment?
Researchers conducting ongoing research projects are required to file e2 continuous research enrollment.
How to fill out e2 continuous research enrollment?
E2 continuous research enrollment can be filled out online through the designated platform.
What is the purpose of e2 continuous research enrollment?
The purpose of e2 continuous research enrollment is to ensure that research data is up to date and accurate.
What information must be reported on e2 continuous research enrollment?
Information such as project updates, new findings, and any changes in research methodology must be reported on e2 continuous research enrollment.
Fill out your e2 continuous research enrollment online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

e2 Continuous Research Enrollment is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.


















