Get the free Department Clinical Research Research - lei org
Show details
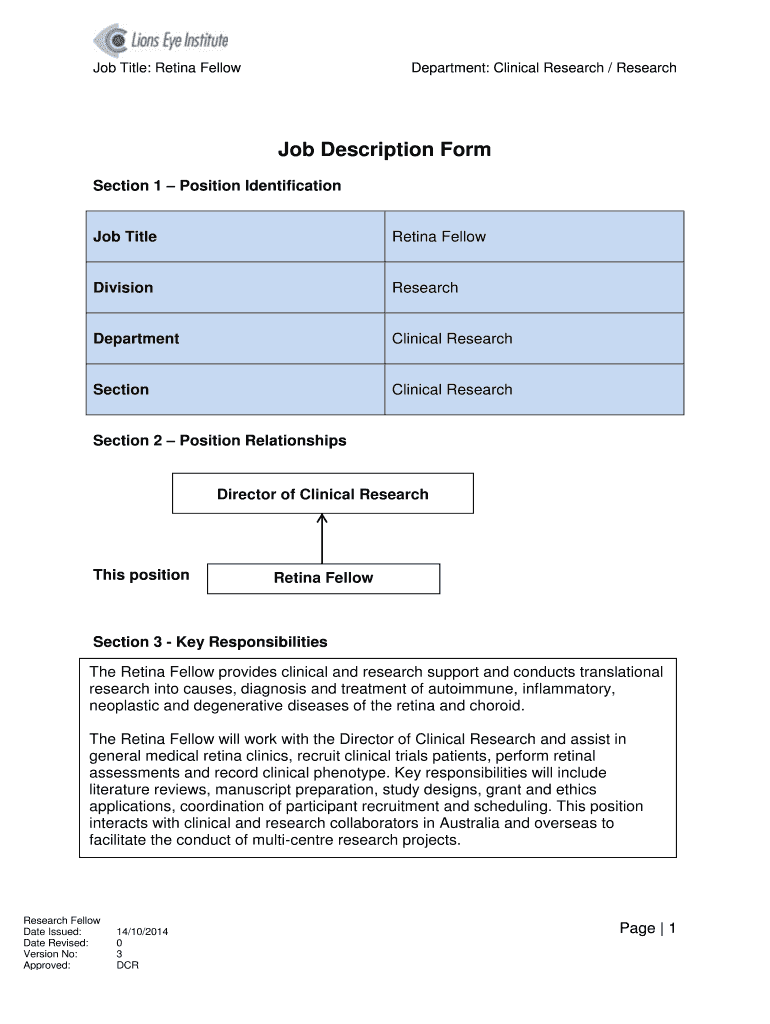
Job Title: Retina Fellow Department: Clinical Research / Research Job Description Form Section 1 Position Identification Job Title Retina Fellow Division Research Department Clinical Research Section
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign department clinical research research

Edit your department clinical research research form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your department clinical research research form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit department clinical research research online
Here are the steps you need to follow to get started with our professional PDF editor:
1
Create an account. Begin by choosing Start Free Trial and, if you are a new user, establish a profile.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit department clinical research research. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Get your file. Select your file from the documents list and pick your export method. You may save it as a PDF, email it, or upload it to the cloud.
It's easier to work with documents with pdfFiller than you can have ever thought. You can sign up for an account to see for yourself.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out department clinical research research

How to fill out department clinical research research:
01
Start by gathering all the necessary information related to the clinical research department. This may include details about the department's goals, objectives, team members, resources, and any ongoing research projects.
02
Assess the current state of the department and identify any areas that require improvement or additional research. This could involve evaluating the efficiency of processes, reviewing existing protocols, or identifying potential research gaps.
03
Collaborate with the department's team members and stakeholders to set clear objectives for the research. Define the specific research questions or hypotheses that need to be addressed and develop a comprehensive research plan accordingly.
04
Determine the appropriate research methodology and design for conducting the research. This may involve selecting the appropriate study design, data collection methods, sample size calculations, and statistical analyses tools.
05
Develop a detailed timeline for the research project, outlining key milestones and deadlines for each stage of the research process. This will help ensure that the research is conducted in a timely manner and according to the predefined schedule.
06
Obtain any necessary approvals or permissions required for conducting the research. This may involve seeking ethical approval, obtaining consent from participants, or securing any necessary funding or resources.
07
Collect and analyze the data in accordance with the research plan. Ensure that all data is accurately recorded, stored securely, and analyzed using appropriate statistical techniques or software.
08
Interpret the research findings and draw conclusions based on the data analysis. This may involve identifying any significant results, discussing the implications of the findings, and making recommendations for future research or improvements to the department's practices.
09
Document the entire research process, including methods used, results obtained, and any limitations or challenges faced during the research. This documentation will serve as a valuable resource for future reference and may also be required for publishing research papers or reports.
10
Finally, share the research findings with relevant stakeholders, such as departmental leadership, team members, or external collaborators. Present the findings in a clear and concise manner, highlighting key takeaways and recommendations for further action.
Who needs department clinical research research?
01
Clinical research departments within healthcare institutions or organizations can benefit from conducting department clinical research research. By conducting this research, they can assess their current practices, identify areas for improvement, and contribute to the advancement of clinical research in their respective fields.
02
Researchers and scientists who are interested in studying the operations and effectiveness of clinical research departments can also find value in department clinical research research. This type of research can provide insights into the factors that contribute to the success or challenges of these departments, and help guide future research in this area.
03
Regulatory bodies and policymakers involved in healthcare and research regulations may also be interested in department clinical research research. The findings from such research can inform the development or modification of policies and guidelines related to clinical research, and ensure that it is conducted in an ethical and effective manner.
Fill
form
: Try Risk Free






For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my department clinical research research in Gmail?
The pdfFiller Gmail add-on lets you create, modify, fill out, and sign department clinical research research and other documents directly in your email. Click here to get pdfFiller for Gmail. Eliminate tedious procedures and handle papers and eSignatures easily.
Can I create an eSignature for the department clinical research research in Gmail?
It's easy to make your eSignature with pdfFiller, and then you can sign your department clinical research research right from your Gmail inbox with the help of pdfFiller's add-on for Gmail. This is a very important point: You must sign up for an account so that you can save your signatures and signed documents.
Can I edit department clinical research research on an Android device?
Yes, you can. With the pdfFiller mobile app for Android, you can edit, sign, and share department clinical research research on your mobile device from any location; only an internet connection is needed. Get the app and start to streamline your document workflow from anywhere.
What is department clinical research research?
Department clinical research research refers to the process of conducting research studies in a clinical setting to evaluate the safety and efficacy of new medical treatments or interventions.
Who is required to file department clinical research research?
Researchers, institutions, or organizations conducting clinical research studies are required to file department clinical research research.
How to fill out department clinical research research?
Department clinical research research should be filled out by providing detailed information about the research study, including its purpose, methodology, participants, and potential risks and benefits.
What is the purpose of department clinical research research?
The purpose of department clinical research research is to ensure that research studies are conducted ethically and in compliance with regulatory requirements to protect the rights and safety of research participants.
What information must be reported on department clinical research research?
Department clinical research research must include information about the study protocol, informed consent process, data management plan, adverse event reporting procedures, and conflict of interest disclosures.
Fill out your department clinical research research online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Department Clinical Research Research is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.




















