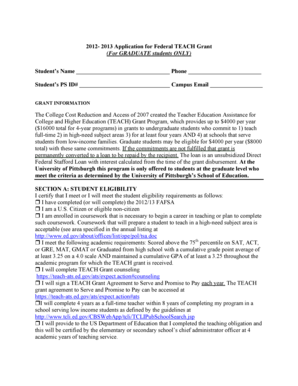
Get the free Provincial Surveillance Protocol for Clostridium difficile infection - health gov nl
Show details
This document outlines the provincial surveillance protocol for monitoring Clostridium difficile infections (CDI). It includes definitions, roles, responsibilities, and procedures for reporting and
We are not affiliated with any brand or entity on this form
Get, Create, Make and Sign provincial surveillance protocol for

Edit your provincial surveillance protocol for form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your provincial surveillance protocol for form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit provincial surveillance protocol for online
Use the instructions below to start using our professional PDF editor:
1
Check your account. If you don't have a profile yet, click Start Free Trial and sign up for one.
2
Prepare a file. Use the Add New button. Then upload your file to the system from your device, importing it from internal mail, the cloud, or by adding its URL.
3
Edit provincial surveillance protocol for. Replace text, adding objects, rearranging pages, and more. Then select the Documents tab to combine, divide, lock or unlock the file.
4
Get your file. Select the name of your file in the docs list and choose your preferred exporting method. You can download it as a PDF, save it in another format, send it by email, or transfer it to the cloud.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out provincial surveillance protocol for

How to fill out Provincial Surveillance Protocol for Clostridium difficile infection
01
Obtain the Provincial Surveillance Protocol for Clostridium difficile infection form from the relevant health authority or website.
02
Review the instructions carefully to understand the requirements for data submission.
03
Fill out the identification section with the facility name, address, and contact information.
04
Record the patient demographic information including age, gender, and admission date.
05
Document details of the Clostridium difficile infection, including diagnosis date and laboratory test results.
06
Include treatment details such as medication administered and duration of treatment.
07
Indicate infection source and any outbreak involvement, if applicable.
08
Sign and date the completed form to certify the accuracy of the information provided.
09
Submit the completed protocol to the designated public health authority as per the guidelines provided.
Who needs Provincial Surveillance Protocol for Clostridium difficile infection?
01
Healthcare facilities required to track and report Clostridium difficile infections.
02
Infection prevention and control practitioners.
03
Public health officials monitoring infection trends for epidemiology purposes.
Fill
form
: Try Risk Free






People Also Ask about
What does the C stand for in C. difficile?
Clostridioides difficile [klos–TRID–e–OY-dees dif–uh–SEEL], formerly known as Clostridium difficile and often called C. difficile or C. diff, is a bacterium (germ) that causes diarrhea and colitis. Colitis is an inflammation of the colon.
What is the gold standard for C. diff treatment?
Treating Recurrent C. Guidelines recommend oral metronidazole or vancomycin for the first recurrence of mild-moderate CDI15,16. Vancomycin is recommended therapy for any subsequent recurrences.
How to monitor for C. diff?
Diagnosing Clostridium Difficile Infections Stool Test. The simplest way to detect C. Blood Test. A blood test can reveal high levels of white blood cells, a sign of infection. Colonoscopy or Sigmoidoscopy. If you have severe symptoms of C. CT Scan.
What is the protocol for C. diff treatment?
However, CDI should usually be treated with an appropriate course (about 10 days) of treatment, including oral vancomycin or fidaxomicin. After treatment, repeat C. diff testing is not recommended if the patient's symptoms have resolved, as patients often remain colonized.
What are the 4C antibiotics?
The term '4C antimicrobials' refers collectively to four broad-spectrum antibiotics, or groups of antibiotics: co-amoxiclav, cephalosporins, fluoroquinolones and , The use of simple generic antibiotics and the avoidance of these broad- spectrum antibiotics preserves them from resistance and reduces the risk
What are the 4 C's for Clostridium difficile?
Methods: As a result guidelines advise treatment with broad spec- trum antibiotics which include the '4C's' (, cephalosporins, co-amoxi- clav and ) which are associated with a higher risk of C. difficile infection.
What are the 4 C's for C. diff?
These antibiotics are all part of the '4 C's' (, cephalosporins, co-amoxiclav and cip- rofloxacin) which are associated with a higher risk of C. difficile infection. Often two or more antibiotics are prescribed at the same time and in the case of osteomyelitis for a minimum course of 4–6 weeks (17–21).
What are preventative measures for Clostridium difficile?
Clostridioides (Clostridium) difficile Prevention Practice good hand hygiene. Practice good hand hygiene. Cleaning surfaces, spills, and accidents. Exclusion Policies.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
What is Provincial Surveillance Protocol for Clostridium difficile infection?
The Provincial Surveillance Protocol for Clostridium difficile infection is a standardized system designed to monitor, report, and analyze cases of Clostridium difficile infection to minimize its impact on public health.
Who is required to file Provincial Surveillance Protocol for Clostridium difficile infection?
Healthcare facilities, including hospitals and long-term care facilities, that diagnose or treat patients with Clostridium difficile infection are required to file the Provincial Surveillance Protocol.
How to fill out Provincial Surveillance Protocol for Clostridium difficile infection?
To fill out the Provincial Surveillance Protocol, healthcare providers must collect patient data including demographics, laboratory results, clinical information, and treatment details, and then enter this information into the designated reporting system as per the guidelines.
What is the purpose of Provincial Surveillance Protocol for Clostridium difficile infection?
The purpose of the Provincial Surveillance Protocol is to enhance the understanding of Clostridium difficile infection's incidence, facilitate timely intervention, improve infection control measures, and ultimately reduce morbidity and mortality associated with the infection.
What information must be reported on Provincial Surveillance Protocol for Clostridium difficile infection?
The information that must be reported includes patient identification details, diagnosis date, laboratory test results, treatment administered, outcome of the infection, and any relevant clinical data that could impact infection transmission.
Fill out your provincial surveillance protocol for online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

Provincial Surveillance Protocol For is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.